
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
please answer all 6-9
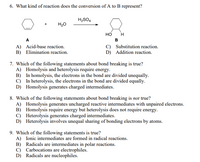
Transcribed Image Text:6. What kind of reaction does the conversion of A to B represent?
H2SO4
H20
но
A
B
A) Acid-base reaction.
B) Elimination reaction.
C) Substitution reaction.
D) Addition reaction.
7. Which of the following statements about bond breaking is true?
A) Homolysis and heterolysis require energy.
B) In homolysis, the electrons in the bond are divided unequally.
C) In heterolysis, the electrons in the bond are divided equally.
D) Homolysis generates charged intermediates.
8. Which of the following statements about bond breaking is not true?
A) Homolysis generates uncharged reactive intermediates with unpaired electrons.
B) Homolysis require energy but heterolysis does not require energy.
C) Heterolysis generates charged intermediates.
D) Heterolysis involves unequal sharing of bonding electrons by atoms.
9. Which of the following statements is true?
A) Ionic intermediates are formed in radical reactions.
B) Radicals are intermediates in polar reactions.
C) Carbocations are electrophiles.
D) Radicals are nucleophiles.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Wrong answerarrow_forwardCan you show how to out this calculation in the calculator? Everytime I do it, I get a different answer.arrow_forwardChemistry 0.1237 (±0.0001) g of sodium carbonate (Na2CO3) required 22.46 (±0.02) cm3 of aqueous HCl for complete neutralisation. Determine the concentration of the HCl in mol dm–3 together with its uncertainty in mol dm–3 to an appropriate number of digits. You may assume the error in the molar mass of Na2CO3 is negligible.arrow_forward
- The Ksp of Al(OH)3 is 4.6*10-33, and the Ksp of Zn(OH)2 is 3*10-17 1) Can Al3+ be separated from Zn2+ by the addition of an NaOH solution to an acidic solution that contains 0.350 M Zn2+ and 0.150 M Al3+ ? If can't leave answer fields in parts 2, 3 empty. (Answer as yes or no) 2) At what hydroxide ion concentration will the second cation precipitate? (Answer must be mol/L) 3) What is the concentration of the other cation at that hydroxide ion concentration? (Answer must be mol/L)arrow_forwardOne of the most important applications of ion exchange is the separation of large molecules from small molecules? 1- true 2- Errorarrow_forwardanalyte concentration(C)(mg/ml) injection volume (ul) elution time (time) peak DAD signal(mAU) caffeine 1 1 4.67 302.85 aspartame 5 1 7.53 15.83 benzoic acid 1 1 8.14 89.98 saccharin 1 1 1.91 84.86 mixture(add everything above with 1:1:1:1 ratio) 1 4.47 69.58 How to get the concentration of the mixture in this case?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY