
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
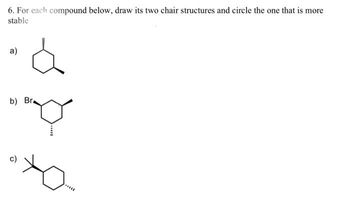
Transcribed Image Text:6. For each compound below, draw its two chair structures and circle the one that is more
stable
a)
á
b) Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6. Draw the two chair conformations of the molecule below. Which is the most stable? HO.arrow_forward1. Compound 1 is one isomer of 1,3-dicholoro-2,4,6-triisopropylcyclohexane. a) Draw both chair conformations of compound 1. State which is more stable. (An isopropyl group should be treated as 'larger' than a chloro group.) b) Draw the most stable achiral stereoisomer of this compound, in its most stable conformation. c) Draw the most stable chiral stereoisomer of this compound, in its most stable conformation. i-Pr i-Pr 1 i-Pr 'CIarrow_forward5) relationship between the pairs of structures. NOTE: Each term may be used more than Choose the term from the five terms listed below that BEST describes the once and not all terms need be used. Identical Diastereomers Enantiomers Constitutional isomers Not isomers CH3 CH3 ÇI H3C-Br CH3 Br -CI H,C. D-H H3C- Br H- -D Br CH3 -CI ČH3 OH H3C, CHO OHC, OH OH H. HO CHO OHC CH3 H. OH ÓHarrow_forward
- Use flat representation of rings, not chair in the drawing. Determine the most and least stable. Consider the most stable chair for each of these isomers, and then draw the most stable and least stable isomer based on a comparison of the best chair for each one.arrow_forwardFor each compound shown below, draw representations for the cis and trans isomers using a hexagon for the six-membered ring, and wedges and dashes for substituents. [1] [1] cis: [2] cis: or [3] cis: ere to search draw structure... draw structure ... draw structure ... ㅍ Bi [2] trans: trans: trans: draw structure... draw structure... draw structure... lyarrow_forwardLabel each compound as cis or trans. Then draw the second chair conformation.arrow_forward
- 11. Draw the most stable chair form of the given molecule.arrow_forward3. Draw both chair conformations of the six membered rings below and circle the lowest energy conformation. If they are equal in energy circle both structures. Draw out all of the H atoms on your chair structure and clearly indicate the directionality for all of the H and non-H substituents. a) b) c) d) Bri Ph,,, OHarrow_forward3) Draw both chair conformations of the six membered rings below and circle the lowest energy conformation. If they are equal in energy circle both structures. Draw out all of the H atoms on your chair structure and clearly indicate the directionality for all of the H and non-H substituents. a) CH3 CH3 CH3 b) d) H3C e) HO HO OH OH OH OHarrow_forward
- (a) Using Newman projections, draw all staggered and eclipsedconformations that result from rotation around the bond highlighted in red in each molecule; (b) draw a graph of energy versus dihedral angle for rotation around this bond.arrow_forwardCan you please explain why in the first one, its just a bondline diagram and the other has wedges and an enantiomer.arrow_forwarddraw newman projection for the indicated bond. put largest groups anti to each other.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning