
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
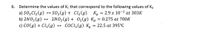
Transcribed Image Text:6. Determine the values of K. that correspond to the following values of Kp
a) SO2C1,(g)
b) 2NO2(g) → 2NO2(g)+ 02(g) Kp = 0.275 at 700K
c) CO(g) + Cl,(g) → COCI,(g) K = 22.5 at 395°C
SO2(g) + Cl2(g)
K, = 2.9 x 10-2 at 303K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 21. At 1000 K, initially pure NO2(g) decomposes according to 2 NO2(g) 2 NO(g) + O2(g) with an equilibrium constant, K = 158, when pressures are expressed in bar. At equilibrium, the partial pressure of O2 = 0.75 bar. What are the partial pressures (in bar) of NO(g) and NO2(g), respectively, at equilibrium at 1000 K? = A) 0.250, 1.0 × 10-¹ B) 0.75, 4.0 × 104 C) 0.125, 1.0 × 10-¹ D) E) 1.50, 1.0 × 10-¹ 1.50, 4.0 × 104arrow_forwardBe sure to answer all parts. The equilibrium constant K, for the reaction I(g) 5 21(g) is 3.75 x 10 at 726°C. Calculate K, and Kp for the equilibrium 21(g) S1(g) at the same temperature. K.= X 10 %3D (Enter your answer in scientific notation.) Kp = %3Darrow_forwardI need the answer as soon as possiblearrow_forward
- For the reaction N2(g)+3H2(g)⇌2NH3(g) Kp = 2.60×10−3 at 290. ∘C . What is Kc for the reaction at this temperature?arrow_forwardThe Kp for the reaction A (g)=2 B (g) is 0.0830. What is Kp for the reaction 2 A (g)=4 B (g)?arrow_forwardAt a given temperature, the system N₂(g) + O₂(g) ≈ 2NO (g) is at equilibrium with Kp = 4.15.The system has the following partial pressures PN 2 1.23 atm and PNO = 5.35 atm. Determine the partial pressure of the oxygen gas present in this equilibrium mixture. -arrow_forward
- The reaction C(s) + 2 H₂(g) = CH₂(g) has Kp = 0.263 at 1000. K. Calculate the total pressure at equilibrium when 4.305 g of H₂ and 22.06 g of C(s) are placed in a 9.08 L flask and heated to 1000. K. Ptotal = Calculate the total pressure when 4.305 g of H₂ and 7.404 g of C(s) are placed in a 9.08 L flask and heated to 1000. K. Ptotal = atm atmarrow_forwardFor the equilibrium N2(g)+O2 <-->2NO(g), Kp = 0.0017 at 2300 K. At a given point, the partial pressures of the gases are PN2=PO2 = 0.660 atm and PNO= 0.0272atm. Which statment below is true? a. Q < K, so the reaction will continue to make more products. b. Q > K, so the reaction will consume products to make more reactants. c. Q = K, so the system is at equilibrium. d. The value of K will decrease until it is equal to Q. Show your work:arrow_forwardConsider the endothermic decomposition reaction of chlorine monofluoride at 1031.0 K. 2 CIF(g) - Ch(g) + F2(g) The equilibrium constant at 1031.0 Kis K= 2.52x106.arrow_forward
- 2. Use the following information to calculate Kp and Ke for each reaction at 1000 K. CO2(g) + C(s) = 2C0(g) Kp = 1.50 at 1000 K a. 2c0(g) = CcO2(g) + C(s) 1 b. ½ CO2(g) + ½ C(s) = CO(g) c. 4C0(g) = 2CO2(g) + 2C(s)arrow_forwardThe following equilibrium constants were determined at 1123 K: Kp=7.79 × 10 Kp"=1.67 × 10² 2CO(g)C(s) + CO₂(g) COC1₂(g) CO(g) + Cl₂ (g) Part 1 of 2 Calculate the equilibrium constant at 1123 K for the reaction: 2COC1₂ (g) C(s) + CO₂(g) + 2Cl₂ (g) Round your answer to 3 significant digits. Part 2 of 2 n x10 = Kp² X Write the equilibrium constant expression: X Olo 15arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY