
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Analyze and interpret the phase diagram of water and carbon dioxide. Briefly, elaborate your answer to each of the following questions:
6. At normal temperature and pressure, at what phase does water exist?
7.-8. At what pressure and temperature will CO2 coexist in three distinct phases of matter?
9. At what temperature is required to have CO2 in solid form?
10. At normal temperature and pressure, at what phase does CO2 exist?
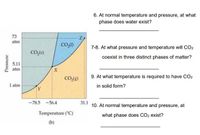
Transcribed Image Text:6. At normal temperature and pressure, at what
phase does water exist?
73
atm
7-8. At what pressure and temperature will CO2
CO,(s)
coexist in three distinct phases of matter?
5.11
atm
9. At what temperature is required to have CO2
CO.(g)
1 atm
in solid form?
TY
-78.5 -56.4
31.1
10. At normal temperature and pressure, at
Temperature ("C)
what phase does CO2 exist?
(b)
Pressure
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 9. Consider the molecule of palmitic acid that is shown below. Draw a figure to show the behaviour of this molecule when it is added to an aqueous solution in high concentration. In your answer, describe what motivates the behaviour of palmitic acid in aqueous solution. HO,arrow_forward18. Name two ions associated with Group 1 cations, and explain what is the difference between Group 1 cations, and how do they differ from Group 4 cations? Give examples. 19. How can PbCl2 be separated from AgCl and Hg2Cl2arrow_forwardThe black “smoke” that flows out of deep ocean hydrothermal vents is made of insoluble metal sulfides suspended in seawater. Of the following cations that are present in the water flowing up through these vents, which ones could contribute to the formation of the black smoke? A. Pb^2+ B. Mg^2+ C. Fe^2+ D. Na^+ E. Li^+ F. Zn^+ G. Ca^2+ H. Mn^2* I. Cu^2+arrow_forward
- How do the reactions of the soap solution with calcium, iron and magnesium ions mimic what happens when soap is used in hard water? Write the equation, including the product formula, for the reaction of soap with calcium ions. Describe common observations around the home due to the reaction of soap with hard water.arrow_forward“Hard” water contains about 2.0 x 10-3 moles Ca2+ per Liter. Calculate the maximum concentration of fluoride that could be present in hard water. Ksp = 4.0 x 10-11arrow_forwardReview the Safety Data Sheet (SDS) for potassium hydrogen phthalate, known as KHP. Classify each statement about KHP as true or false. KHP is incompatible with strong oxidizing agents. KHP is flammable. KHP is a carcinogen. KHP must be stored under an inert gas. Eye protection and gloves are recommended when using KHParrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY