
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
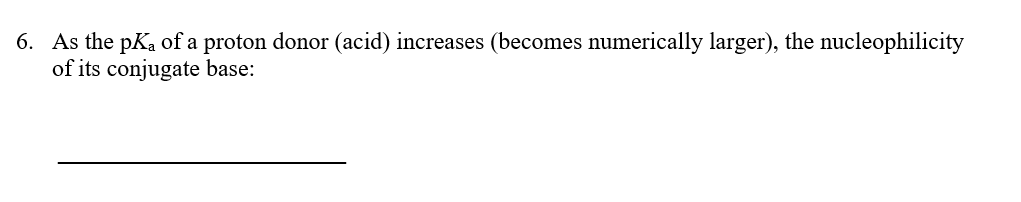
Transcribed Image Text:6. As the pKa of a proton donor (acid) increases (becomes numerically larger), the nucleophilicity
of its conjugate base:
Expert Solution
arrow_forward
Step 1
1) High pKa value means weak acid,
low pKa value means strong acid.
2) Strong acid have weak conjugate base
and Weak acid have strong conjugate base.
And a conjugate base is always a stronger nucleophile.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- What is the pKa value of molecule C? OH soni +arrow_forward10) What is the pK of a weak acid with a Kof 4.7 X 10“?arrow_forwardWhen ammonia is placed in water, the following two reactions (1 or 2) occur to a certain extent. Reaction 1: NH3 (aq) + H2O (l)⇄⇄ NH4+ (aq) + OH- (aq) pKb = 4.75 Reaction 2:NH3 (aq) + H2O (l)⇄⇄ NH2- (aq) + H3O+ (aq) pKa = 35 Which reaction is most likely to occur? a.) Both equally b.) More info needed c.) Reaction 1 d.) Reaction 2arrow_forward
- How do Lewis bases/acids affect the pH of a solution? If they are bases, they should cause the OH- concentration to increase or the H+ concentration to decrease. If they are acids, the opposite. However, I don't see how they can do that if they are simply electron acceptors/donors. Can someone explain the mechanism?arrow_forwardpKal pK₂2 Phosphoric acid, H, PO, (aq), is a triprotic acid, meaning that one molecule of the acid has three acidic protons. Estimate the pH and the concentrations of all species in a 0.300 M phosphoric acid solution. 2.16 7.21 [H, PO₁] = M [H*] = [H₂PO¡] = M [OH-] = [HPO ] = M pH = [PO] = M PK₂3 12.32 M Marrow_forward12. Bicine is an organic compound used as a buffering agent. It is one of Good's buffers and has a pKa of 8.354 at 25 °C. The molecular formula of bicine is C6H13NO4. Bicine can react with Ba(OH)₂ according to the following reaction. 2 C6H13NO4 + Ba(OH)2 → Ba(C6H12NO4)2 + 2 H₂O It took a student worker 3 steps to make a buffer solution. First, a bicine solution is made by dissolving 37.47 grams bicine in enough water. The total volume is 200.00 mL. Then 250.00 mL Ba(OH)2 solution is prepared by adding 36.57 grams Ba(OH)2 solid and enough water. At the end, a buffer solution is made by mixing 157.89 mL of the above bicine solution and 89.32 mL of the above Ba(OH)₂ solution. A. B. Determine the equilibrium concentration of bicine in the buffer solution. Please provider your answer below. Determine the equilibrium concentration of Ba(C6H₁2NO4)2 in the buffer solution. Please provider your answer below. Determine the pH of the buffer solution. pH = Please provider your answer below. 0² 0₂…arrow_forward
- Will 3-methylhexanoic acid be positive or neutral with an pH of 6.38?arrow_forwardConjugate acid base 2) Consider the reaction B:(-) + H3O+ → BH(+) + H2O. For the following named bases: 1) draw the structure of the base, 2) draw the structure of the conjugate acid, 3) give the name of the conjugate acid, and 4) give the approximate pKa of the conjugate acid (±1 pKa unit). For the purposes of answering this question you may use pKa values evenly divisible by 5. Benzoate anion Cyclopentadienide anion Diethyl amine Diethylamide anion pKa pKa pKa pKaarrow_forward6. For the ionization of phenylacetic acid, C6H5CH₂CO₂H + H₂O = H3O+ + C6H5CH₂CO₂ K₂ = 49 × 10-5 (a) What is [C6H5CH₂CO₂] in 0.186 M C6H3CH₂CO₂H? (b) What is the pH of 0.121 M C6H3CH₂CO₂H?arrow_forward
- 12. Bicine is an organic compound used as a buffering agent. It is one of Good's buffers and has a pKa of 8.354 at 25 °C. The molecular formula of bicine is C6H13NO4. Bicine can react with Ba(OH)2 according to the following reaction. 2 C6H13NO4 + Ba(OH)2 → Ba(C6H12NO4)2 + 2 H₂O It took a student worker 3 steps to make a buffer solution. First, a bicine solution is made by dissolving 37.47 grams bicine in enough water. The total volume is 200.00 mL. Then 250.00 mL Ba(OH)2 solution is prepared by adding 36.57 grams Ba(OH)2 solid and enough water. At the end, a buffer solution is made by mixing 157.89 mL of the above bicine solution and 89.32 mL of the above Ba(OH)2 solution. A. B. C. Determine the equilibrium concentration of bicine in the buffer solution. Please provider your answer below. A of Ba(C6H12NO4)2 in the buffer solution. Check answer Determine the equilibrium concentration M Please provider your answer below. A pH = Please provider your answer below. A $ Check answer…arrow_forwardQ16. Identify the two acid/conjugate base and base/conjugate acid pairs in the following acid/base reaction: ] Using Evan's pKa table, assign a pka value to the acid and conjugate acid species on the opposite side of the reaction arrow. Use the assigned pka values to predict if the preferred direction for this reaction is forward or backwards. Et;N or + + MeO OMe Et,NH MeO OMearrow_forwardConjugate base acid Iso-Butyronitrile pka_ → 1) Consider the reaction AH(+) + H₂O A:+H3O+. For the following named acids: 1) draw the structure of the acid, 2) give the approximate pKa of the conjugate acid (±1 pKa unit), 3) give the name of the conjugate base, and 4) draw the structure of the conjugate base. For the purposes of answering this question you may use pKa values evenly divisible by 5. N-methylimidazolium cation pka_ Triethyl phosphonoacetate pKa _ acid N-Ethylcarbamic pka_arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY