
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
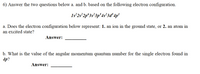
Transcribed Image Text:6) Answer the two questions below a. and b. based on the following electron configuration.
Is 2s 2p°3s*3p°4s*3d* 4p'
a. Does the electron configuration below represent: 1. an ion in the ground state, or 2. an atom in
an excited state?
Answer:
b. What is the value of the angular momentum quantum number for the single electron found in
4p?
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A.) What is the grounds the electron configuration of 15p. B.) Show the orbital diagram. C.) Write the four quantum numbers associated with the last electron. D.) Is it paramagnetic or diamagnetic?arrow_forwardQuantum Numbers A. Quantum Numbers and Sublevels: Insert the appropriate values in the chart below. Principal Quantum # (n) 1 2 3 4 Possible Values for Angular Momentum Quantum # (1) T Letter Designation for each value of / 11 Total Number of Sublevels per Levelarrow_forwardPlease don't provide handwritten solution ....arrow_forward
- 4. Electrons must gain energy in order to move from lower energy levels to higher energy levels. When the electron moves back down from a higher energy level to a lower one, how does it "lose" this energy? Where does the energy go? Edit View Insert Format Tools Table 12pt v Paragraph v BIU T?v:arrow_forwardThe following is a diagram of energy states and transitions in the hydrogen atom. ENERGY B ( D E In 1 U Match each of the responses below with the correct arrow from the figure. 1. The emission line with the shortest wavelength. 2. The absorption line with the shortest wavelength. 3. The emission line with the highest energy. 4. The absorption line with the highest energy. 5. The emission line with the lowest frequency. 6. The line corresponding to the ionization energy of hydrogen.arrow_forward9. Calculate the longest wavelength (in um) of light emitted by an excited hydrogen atom in which the electron occupies the energy level n = 6. A) 3.28 B) 1.00 C) 7.46 D) 93.7 E) 2.28arrow_forward
- This is from a study guide for a final, not a graded assignment.arrow_forwardEnergy Level Diagrams and Electron Configurations Worksheet 1. Write the 15 sets of quantum numbers that describe the 15 electrons of P. 2) Write down the quantum number(s) indicated by the following statements. a. The third energy level: b) The 4s sub-shell: c) The orbitals in the 2p subshell: d) An electron in the 5f sublevel: e) The orbitals in the 3f subshell: f) The first energy level: g) The lowest energy subshell in the third energy level: h) The highest energy subshell in the fourth energy level: 3) Write down the maximum number of electrons that can fit into each of the following shells, sub-shells, orbitals, or spins. n = 2, 1 = 0, ml = 0 n= 4, 1 = 2, ml = -1, ms = +2 n = 5, 1 = 3 n = 4, 1 = 3, ml= -2, ms -2 %3D n= 3, 1 = 2, ml = -1 n = 5, 1 = 1 n= 3, 1 = 2, ml = -2, ms = -2 %3D n = 3, 1 = 2, ml = -1 n= 2, 1 = 1, ml =+1, 4) On a separate sheet of paper, draw the orbital diagrams for He, Li, N, Mg, Ar, Fe, Nb, Tb, and U. 5) Write the electron configurations of the following…arrow_forwardQ1/ Select the correct choice: 1. The process of removing an electron from an atom is called a:(Binding) b:(Excitation) c;(nonionizing) d;(ionization) 2. The state of lowest energy is the one in which the atom is normally found and is called a; (excited state). b;(ionizing state) c(ground state) d;(normally state). 3. The energy of the x-ray will be equal to the different between: a(wavelength) b(number of electron) c(Absorption spectroscopy) d(energy levels of the atom)arrow_forward
- $ 4 F In the Bohr model of the hydrogen atom, the electron occupies distinct energy states. One transition between energy states of the hydrogen atom is represented by the picture. R n=∞ V n=4 n=3 n-2 n=1 In this transition an electron moves from the n = level to the n = Energy is in this process. The electron moves I Submit Answer % 5 T G [Review Topics] [References] Use the References to access important values if needed for this question. B Retry Entire Group 9 more group attempts remaining 6 Cengage Learning Cengage Technical Support the nucleus. Y H MacBook Pro N & 7 U J * 8 M | K 9 L I level. O O L P > { Previous [ ? + 11 11 Next Save and Exit } ] deletearrow_forwardPart A Use the orbital-filling diagram to show the electron configuration of phosphorus, P. Be sure to label the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Drag the appropriate labels to their respective targets. Not all targets will be filled. • View Available Hint(s) Reset Help | 1 G2 G1 1s G2 G1 G1 G1 2s 2p G2 G1 3s G2 G1 G1 G1 Зр G2 G1 3d G2 G1 4s 4p 1Larrow_forward101 Chem101 b My Questions | bartleby x + -> app.101edu.co M Apps G M Gmail YouTube Maps a AMAZON Translate O Gflights Case Status Onlin... b Homework Help a... C Get Homework He... > KATAPULK CUBA 23 Agencia Supermar.. Reading List Question 3 of 22 Submit Which of the following is not permitted as a plausible orbital designation according to quantum theory. A) 4f B) 7s C) Зр D) 3f +arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY