
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
balance the equations
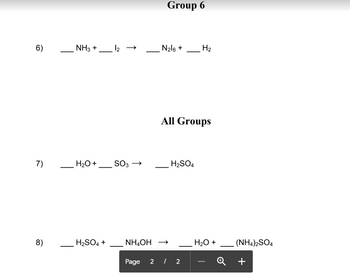
Transcribed Image Text:6)
7)
8)
NH3 +
H₂O +
H₂SO4 +
12 →
SO3 →
Group 6
N₂16 +
All Groups
NH,OH →
H₂SO4
H₂
Page 2 / 2
H₂O +
(NH4)2SO4
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The percent yield for a given reaction is 60%. If the theoretical amount is predicted to be 8.33 g, what was the actual yield for the reaction?arrow_forwardPhosphorus pentachloride can be produced by reacting phosphorus trichloride and chlorine gas. What mass of phosphorus trichloride must be used to produce 127 g of phosphorus pentachloride if the percent yield is 84.8%? You will need to write a balanced equation.arrow_forwardAdipic acid is used in the production of nylon, so it is manufactured in large quantities. The most common method for the preparation of adipic acid is the reaction of cylcohexane with oxygen. Balance the skeleton equation below. (Use the lowest possible whole number coefficients.)arrow_forward
- For the following reaction, 0.162 moles of carbon (graphite) are mixed with 0.501 moles of oxygen gas. What is the formula for the limiting reagent? What is the maximum amount of carbon dioxide that can be produced?arrow_forwardWhich of the balanced chemical equations is consistent with the following pictorial representation of a chemical reaction?arrow_forwardBalance the equationarrow_forward
- why did you not balance the equation?arrow_forward“Magnesium metal is burned in the presence of atmospheric oxygen to form magnesium oxide. Student 1 data Mass Mg 0.1712 g Mass after reaction 0.2886 g Using the given data, calculate the stoichiometric ratio of magnesium ions to oxygen ions in this magnesium oxide sample. Express the ratio as a decimal. Include four significant figures.”arrow_forwardFor the reaction If 2.4 g Ti is reacted with 1.6 g F2 what mass of TiF4 will be produced?arrow_forward
- For the reaction of nitrogen dioxide with water to form nitric acid and nitrogen monoxide, what is the sum of the coefficients of the balanced equation?arrow_forwardgobjxmesyVpHOEI yobixmesyvpHOEB1plef9xyC5Ca9Q15ULF571w.. plef9xyC5Ca9QI5ULF571w... A o O CHEMICAL REACTIONS Identifying the limiting reactant in a drawing of a mixture The drawing below shows a mixture of molecules: key carbon hydrogen nitrogen O sulfur oxygen O chlorine Suppose the following chemical reaction can take place in this mixture: CH (9)+2O,(g) → CO,(g)+2 H,0(g) Of which reactant are there the most initial moles? Enter its chemical formula: Of which reactant are there the least initial moles? Enter its chemical formula: Which reactant is the limiting reactant? Enter its chemical formula: Explanation Check 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use P 7111 W OL IOarrow_forwardBalance the following equation using the smallest whole number coefficients: SiO2 +C SiC + CO The coefficient for carbon is:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY