
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
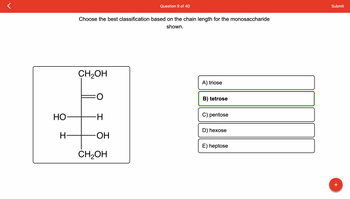
Transcribed Image Text:HO
H-
Choose the best classification based on the chain length for the monosaccharide
shown.
CH₂OH
-H
OH
Question 9 of 40
CH₂OH
A) triose
B) tetrose
C) pentose
D) hexose
E) heptose
Submit
+
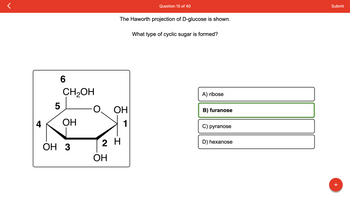
Transcribed Image Text:4
5
6
CH₂OH
ОН
OH 3
OH
The Haworth projection of D-glucose is shown.
ОН
1
2 H
Question 15 of 40
What type of cyclic sugar is formed?
A) ribose
B) furanose
C) pyranose
D) hexanose
Submit
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Assign a name to the following monosaccharide. Use D and Ldesignation. CHO HO H HO H Но- HOH ČH2OHarrow_forwardwhat is the structure digested by and how many reducing sugars are present ОН ОН Но OH HO Но HO- OH HO, The trisaccharide pictured could be completely digested (ie into monomers) by a beta-galactosidase and an alpha-glucosidase an alpha-galactosidase and sucrase lactase and sucrase a beta-fructosidase and lactasearrow_forwardClassify the following monosaccharide. Но H. ОН H- OH H- CH2OH D or L: [Select ] Aldehyde or Ketone: [Select ] Number of Carbons: [ Select ] エエOarrow_forward
- a. Classify this monosaccharide b. Does it have the D or L configuration? c. Specify the type of ring this structure has. d. Is the configuration of the anomeric carbon alpha or beta?arrow_forward17. Draw the 2-amino sugar and the aldonic acid that can be formed from the following monosaccharide. || C-H H- -OH H -OH HO- -H H -OH CHOH D-gulosearrow_forwardDraw the structure that formed between alpha-L-Erythrose with the amino acid Asparagine and label the type of bond that is formed.arrow_forward
- 10 Mark the following statements as true or false, explaining in a few words the reasons for your choice. a) The disaccharide in question 9 is a reducing sugar. b) The reaction of D-fructose with Tollens reagent gives two aldonic acids. c) Morphine and codeine are isomeric compounds. d) Adenine, guanine and cytosine are all purine bases. e) Prostaglandins PGB and PGC have a single O atom in their structure.arrow_forwardCorrectly name this monosaccharide, including D or L designation. Н. H НО- НО- H OH H H -OH CH₂OHarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY