
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:50 g of copper is heated to increase its temperature by 100C. If the same amount of heat is applied
to 10 g of water, its rise in temperature would be (Specific heat of copper = 420 J/k °C)
%3D
O 8°C
O 5°C
O 7°C
O 6°C
Expert Solution
arrow_forward
Step 1
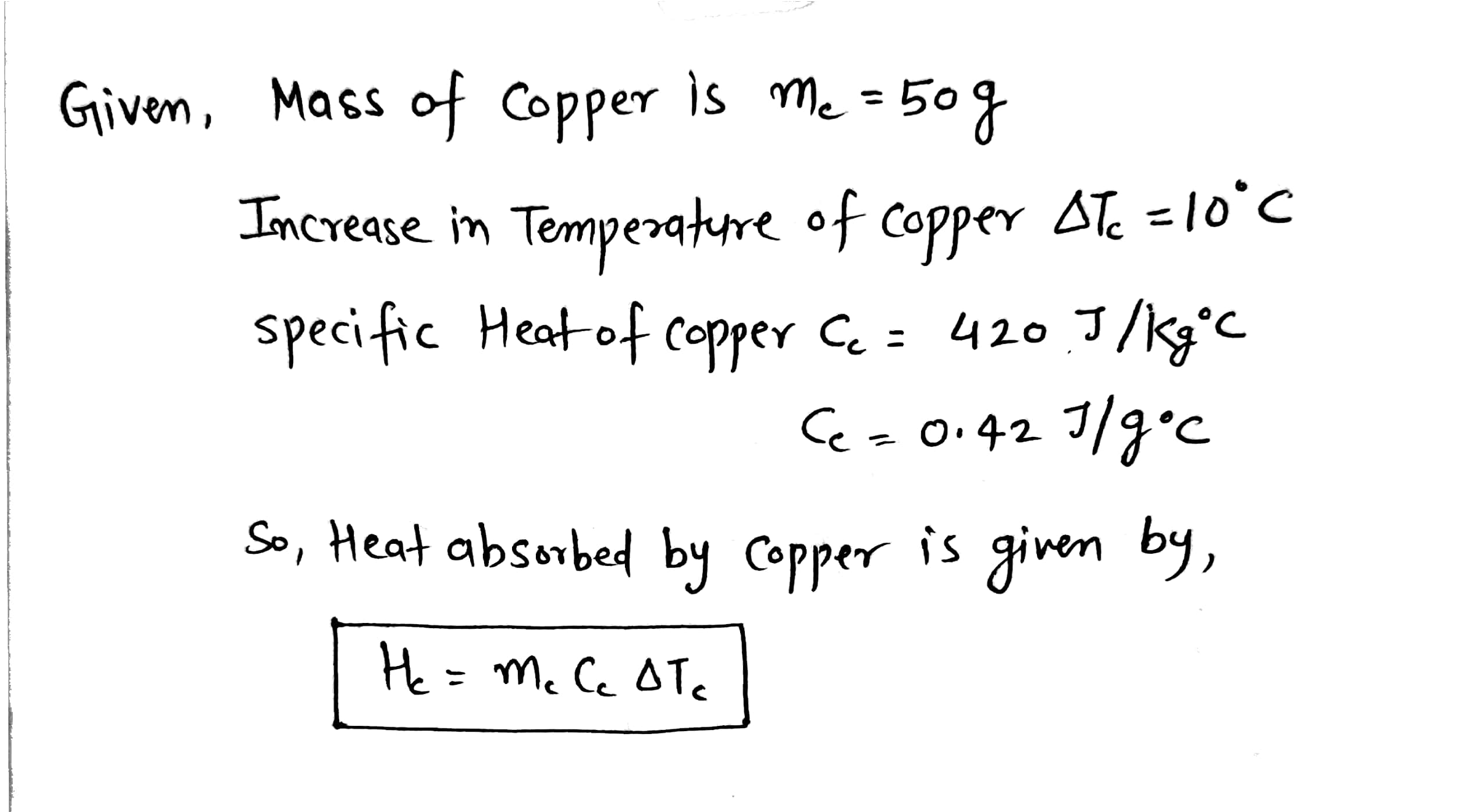
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Calculate the latent heat of vapourisation for water at 54°C using the data contained in the table below. Temperature /°C p(sat)/kPa Saturated Liquid Volume/cm^3g^-1 Saturated Vapour Volume/cm^3g^-1 53 14.29 1.014 10490 54 15.00 1.014 10020 55 15.74 1.015 9577.9arrow_forwardIn a well-insulated bucket of negligible heat capacity, 0.32 Kg of water at 27 °C is mixed with ice at 0 °C. What's the maximum mass of ice that would melt in the bucket, if any? Cwater = 4186 J/Kg °C Lf, water = 3.35 x 105 J/Kgarrow_forwardPlease asaparrow_forward
- A 117-g cube of ice at 0°C is dropped into 1.0 kg of water that was originally at 79°C. What is the final temperature of the water after the ice has melted?arrow_forwardHow much heat energy must be removed from 0.10 kg of oxygen with a temperature of 22 °C in order for the oxygen to liquefy at -183 °C? (Co = 913 J/kg°C, Ly = 2.13 x 105 J/kg and oxygen liquefies as -183 °C) O 5 x 10^4 J O 9 x 10^4 J O 1 x 10^4 J O 4 x 10^4Jarrow_forwardA 365-g sample of an unknown (nongaseous) material experiences a 16.0°C increase in temperature after absorbing 9.93 ✕ 103 J of energy. (a) What is the specific heat of this material? J/(kg · °C)(b) Which substance listed in the specific heat and molar specific heat table best matches the specific heat of this unknown material? mercurysilver brassgraniteglassaluminumwoodseawaterarrow_forward
- Steam at 100°C is added to ice at 0°C. (a) Find the amount of ice melted and the final temperature when the mass of steam is 11 g and the mass of ice is 58 g. ?g ?°C (b) Repeat with steam of mass 3.7 g and ice of mass 58 g. ? g ?°Carrow_forwardWhat quantity of heat (in J) would be required to convert 2.65 mol of a pure substance from a liquid at 40.0 °C to a gas at 113.0 °C? A Cliquid = 1.45 J/mol °C Tboiling = 88.5 °C Cgas = 0.65 J/mol °C AHvaporization = 1.23 kJ/molarrow_forwarda sample of beryllium (1820J/kgC) at 20 degrees celsius into a 0.22kg glass beaker also at 20 degrees celsius. Water of mass 0.3kg and temperature 80 degrees celsius is poured over the beryllium. If the thermal equilibrium temperature of the closed system is 40 degrees celsius, what is the mass of the beryllium?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON