
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
help please
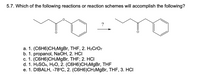
Transcribed Image Text:5.7. Which of the following reactions or reaction schemes will accomplish the following?
а. 1. (С6H6)СH2MgBr, THF, 2. Н2CrO7
b. 1. propanol, NaOH, 2. HCI
с. 1. (С6H6)CН-MgBr, THF; 2. НСІ
d. 1. HaSO4, H20, 2. (С6H6)СHгMgBr, THF
е. 1. DIBALH, -78°C, 2. (C6H6)CH-MgBr, THF, 3. HСІ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- My prof told me that You can interpolate the graph to read the concentration value By interpolating I got the value 2.04 mg/L This is how we do question no 5 But I am little confuse how to do it I resubmitted question with grapharrow_forwardHello can you please help me answer this?arrow_forwardhelp mearrow_forward
- rch ||| O CHEMICAL REACTIONS Predicting the products of a neutralization reaction Predict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced. HNO3 + NaOH → 0 Explanation Ease RK- burial WE burial Check papers 46 H. Kdy go ahead X 09 1/5 Christopher Mitchell Jessica V 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 000 14 GEarrow_forwardWhat would step 1 and 2 be?arrow_forwardTrue or false The first aid for chemicals gotten in eyes or on skin is to wash the eyes or skin immediately with water for at least 15 minutesarrow_forward
- Please complete the calculations related to pH, pOH, [H3O+], and [OH-]. Remember there are special rules related to significant digits when working with pH, pOH, [H3O+], and [OH-]. A sample of household vinegar was measured to have a hydronium ion concentration of 5.8 x 10-3mol/L. Determine the pH of the sample. Hand soap is effective at preventing bacterial contamination. Calculate the hydronium ion concentration in a brand of hand soap with a pH of 10.1Human saliva contains enzymes that start the process of digestion. Human saliva typically has a hydroxide ion concentration of 1.2 x 10-8 mol/L. Determine the pOH of saliva. Human blood is slightly basic. Calculate the hydroxide ion concentration in a sample of blood with a pOH of 6.55arrow_forwarda) Determine the concentration of acetic acid in the 25.00 mL aliquot. b) Determine the concentration of acetic acid in the 10.0mL of vinegar. concentration NaOH = 0.101M Trial 3 Initial NaOH level 35.1 mL Final NaOH level 52.5mL The volume of NaOH used to reach the endpoint 17.4 mLarrow_forwardSuper fast answer super fast!!!!!!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY