
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
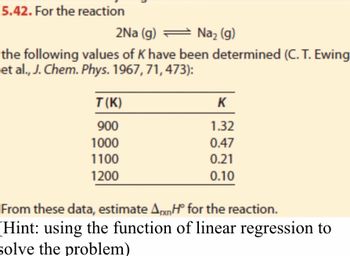
Transcribed Image Text:5.42. For the reaction
2Na (g) = Na₂ (g)
the following values of K have been determined (C. T. Ewing
et al., J. Chem. Phys. 1967, 71, 473):
T(K)
900
1000
1100
1200
K
1.32
0.47
0.21
0.10
From these data, estimate AxH for the reaction.
(Hint: using the function of linear regression to
solve the problem)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- the following thermesdyna mic and partial prassuce data, detormine the Gibbs Energy (AG) for the reaction : Give 2 PeO cg) Clz cq> 2 2 Pe OCI (9) at 298 k in kJ /mol substance AG (KJ/ncol ) P (bar) Pe O cg> - 220.4 0.40 ch (q? Information is missing 0.17 PeOckgs 420.4 0.59arrow_forwardCalculate the change in S when one mole of water is heated from 263 to 283 K given the molar capacities inJ.K-1 , Cp(ice) = 2.09 + 0.126T, Cp(water)=75.3, and change in Hm=6000Jmol-1arrow_forward10mL of an aqueous solution containing 0.020 M RCOOH (pKa = 6.00) is mixed with 10mL of CCI4. The partition coefficient is 3.0. When the pH of the aqueous phase is adjusted to 6.00, 0.012 M of RCOOH is measured in the CCL4 phase. What will be the formal concentration of RCOOH in the aqueous solution if its pH was adjusted to 7.00 before extraction?arrow_forward
- A7arrow_forward(4c) Consider a situation where phenol is partitioned between water and benzene with a partitioncoefficient of 1.35in favour of benzene. Suppose we have 2g of phenol dissolved in 250ml of water. How muchphenol will be extracted into 250ml of benzene with a single extractionand howmuch phenol is left in water?.arrow_forwardSuppose now that argon is added to the mixture in the previous exercise to bring the composition closer to real air, with mole fractions 0.780. 0.210, and 0.0096, respectively. (a) What is the additional change in molar Gibbs energy and entropy at 298 K? (b) Is the mixing spontaneous?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY