
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
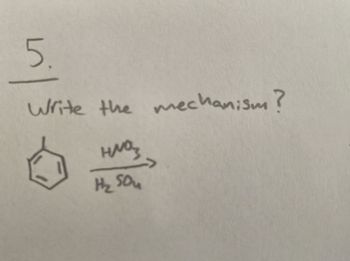
Transcribed Image Text:5.
Write the mechanism?
H₂ Son
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 5. Which of the following statements regarding the reactions A and B (shown below) is the best? 0 heat 0 heat B A . Both reaction A and B are feasible because frontier orbitals of reactants can overlap in-phase, allowing the simultaneous formation of two o bonds. . Both reaction A and B are NOT feasible because frontier orbitals of reactants can NOT overlap in-phase. Only reaction A is feasible because frontier orbitals of reactants can overlap in- phase, allowing the simultaneous formation of two o bonds. Only reaction B is feasible because frontier orbitals of reactants can overlap in- phase, allowing the simultaneous formation of two o bonds.arrow_forward8arrow_forwardPls help ASAParrow_forward
- Drew the mechanismarrow_forwardDraw the step by step reaction mechanism and prerdict products(s) of the following reactionarrow_forwardMechanism: A reaction mechanism for the following reaction is shown below. H+ CEN CEN: N-H || -C-OH H₂O, H* Step 1 wand woled mot ozsm E Step 3 N-H C-OH C=N-H Step2 C=N-H H-O-H H₂O motno vgiene fesrigid onlt to smotnos -C=N-H a) The overall reaction is an example of b) Step 1 is c) Step 3 is d) Draw in the curved arrows for each step. e) Identify the nucleophiles and electrophiles where appropriate. f) Fill in the reaction energy diagram. H₂O: rate determining step to noipojovo hamwell sit 5(emise erit voittons 10) vanas mi sdgin al rainW di of E^ reaction progress →arrow_forward
- What Is the starting materlal of this reaction? Click on a letter A through D to answer. CH,OH H+ cat. А. С. В. OH D.arrow_forwardWhat happens to the rate of this reaction if the concentration of CH3OH is doubled? the n CH3OH tic OCH3 Br A it quadruples B it is cut in half Ci t doubles D it is unchangedarrow_forwardMechanism: A reaction mechanism for the following reaction is shown below. H+ CEN CEN: N-H || -C-OH H₂O, H* Step 1 Step 3 N-H C-OH C=N-H H₂O Step2 .C=N-H H-O-H Semse anls a) The overall reaction is an example of b) Step 1 is c) Step 3 is d) Draw in the curved arrows for each step. e) Identify the nucleophiles and electrophiles where appropriate. f) Fill in the reaction energy diagram. C=N-H H₂O: rate determining step E个 reaction progress →arrow_forward
- Choose the reaction that will not proceed as written. OH H. 1) xs LIAIH4, ether a) H,C 2) H+, H20 NH2 b) H,C H3C HO, H3C CI pyridine OH H+ 1) xs CH3MgBr,ether + NH4+ + CH3CH2OH c) H3C H20 d) H;C NH2 OH OCH2CH3 2) H+, H20 H3C CH3 CH heat O b O a O darrow_forwardWhat is the effect of halving the amount of ethanol for this reaction? Br OH heat the reaction rate decreases by half the reaction rate stays the same А. В. C. the reaction rate doubles D. the reaction rate quadruples A Darrow_forward1c. What is the nucleophile in the following reaction? .Br CH;CO0 Na LO OCCH3 NaBr ld. What is the electrophile in the following reaction? Br CH;COO Na L0OCCH3 NaBr +arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY