
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
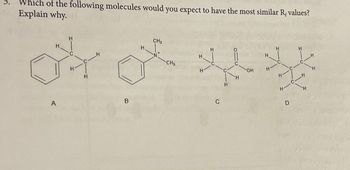
Transcribed Image Text:Which of the following molecules would you expect to have the most similar R₁ values?
Explain why.
H
A
H
H
B
H
CH3
CH3
H
H
OH
H
H
D
H
H
H
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Click on all structures that are enantiomers of the first (leftmost). If no structure qualifies, submit your answer without selecting any structure. CO₂H со н HOH₂C H NH₂ HI H₂N CO₂H SCH2OH H₂N CO₂H CH₂OH HOH₂C H NH₂arrow_forwardFor each organic compound in the table below, enter the locant of the highlighted side chain. compound CH3 | CH₂ - CH₂ - CH - CH₂ CH3 I CH3 CH₂-C — CH3 CH₂ CH3 CH₂ - CH CH3 locant of highlighted side chain × 0 0 0 Śarrow_forwardWhich molecule answers this question (you are not looking for the "?" you are looking for the answer to this question). A B с D H3C H₂ A B H3C- H3C OH Br H₂ H PBr3/Br2 H₂ CH₂OH Br OH ? D BrH₂C H3C- H₂ H₂O H₂ Answer to the question CH₂OH OHarrow_forward
- Please help me with this i am very confused, if I have a current answer it is incorrect and I am just using this to study and I am very confusedarrow_forwardModify methionine to show its zwitterion form.arrow_forwardU D HO: H-C-C-N. | Br Ca Br₂ I CH3 CH3 x (Choose one) ✓ (Choose one) ✓ Sarrow_forward
- How many non-equivalent hydrogens does this molecule have?arrow_forwardDraw 3-methylcyclobutanol. Include all hydrogen atoms. Select Draw Rings 0 H₂C с - T с H₂ H₂C What is wrong with this? H₂ с | CH3 H OH Morearrow_forwardCan i get step by step help with this problem step by step pleasearrow_forward
- For each organic compound in the table below, enter the locant of the highlighted side chain. CH3 CH,— CH,— CH— CH, CH3 1 compound CH₂ | CH,—C− CH,—CH — CH CH3 モー CH3 CH,—CH— CH,—C— CH | CH₂ CH3 CH3 | CH3 locant of highlighted side chain 0 X Sarrow_forwardWhich molecule has the lowest pk₂? (Your answer may include more than one of the choices if the molecules shown have the same value.) H | A B A | C H B H H C Harrow_forwardIn the answer box, type the number of maximum stereoisomers possible for the following compound. A H H COH OH = H C Br H.C OH CHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
