
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:5. What effect, if any, would varying the concentration of both the aqueous species have on the
cell potentials? What about varying the size of the electrodes?
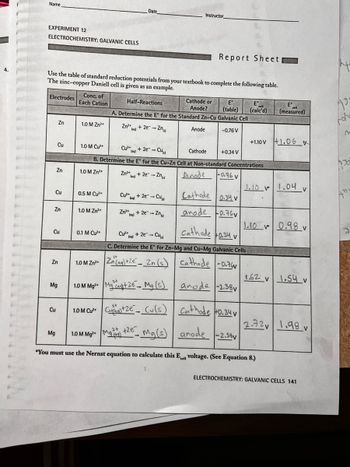
Transcribed Image Text:4.
Name
EXPERIMENT 12
ELECTROCHEMISTRY: GALVANIC CELLS
Electrodes
Use the table of standard reduction potentials from your textbook to complete the following table.
The zinc-copper Daniell cell is given as an example.
Cu
Cu
Zn
Zn
Zn
Cu
Zn
Mg
Cu
Mg
Conc. of
Each Cation
1.0 M Zn²+
0.5 M Cu²+
1.0 M Zn²+
0.1 M Cu²+
Date
1.0 M Zn²+
Zn²+ + 2e-Zn
(ag)
Half-Reactions
A. Determine the E for the Standard Zn-Cu Galvanic Cell
Zn²+
(aq)
1.0 M Cu²+
Cu²+
+2e-Cu
Cathode
B. Determine the E* for the Cu-Zn Cell at Non-standard Concentrations
1.0 M Zn²+
Anode
+ 2e → Zn
Instructor
Cu²+2e-Cu
Zn²+ + 2e → Zn
(aq)
Report Sheet
Cathode or
Anode?
Anode
E
E
(table) (calcd)
-0.76 V
+0.34 V
-0.76V
Cathode 0.34 v
anode 0.76v
Cathode 0.34 v
Cu²+ + 2e - Cul
C. Determine the E for Zn-Mg and Cu-Mg Galvanic Cells
Zn(aq) +2e Zn (s) cathode -0.76
1.0 M Mg²+Mga+2e Mg(s) anode -2.38
+1.10 V
1.10 V
1.0 M Cu²+ +Ze Cu(s) Cathode 40.34 v
2+ +2e
1.0 M Mg²+ M
Mg(s) anode -2.38v
"You must use the Nernst equation to calculate this Ecell voltage. (See Equation 8.)
1.62 v
1.10 0.98 v
Eº
(measured)
2.72v
+1.06 x
1.04 v
1.54 v
1.99 v
V
ELECTROCHEMISTRY: GALVANIC CELLS 141
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the standard cell potential and note whether the reaction is spontaneous under standard conditions. Mg(s) + Cu2+ --> Mg2+ + Cu(s)arrow_forwardQuestions 20 and 21 refer to the following information. Galvanic Cell Cell Diagram E°cell (Volts) Mg | Mg2(1.0 M) || Pb*2(1.0 M) | Pb 1 2.24 Zn | Zn(1.0 M) || Pb*2(1.0 M) | Pb 2 0.63 Mg | Mg*(1.0 M) || Zn*2(1.0 M) | Zn 3 The chemical reaction occurring in first galvanic cell is: Mg + Pb*2 → Mg+2 + Pb 21. If the concentration of Mg+2 is changed from 1.0 M to 3.0 M in the galvanic cells above, what will happen to the observed cell voltages in galvanic cells 1 and 3? (A) The voltage in both galvanic cells 1 and 3 will increase. (B) The voltage will decrease in both galvanic cells 1 and 3. (C) The voltage in galvanic cell 1 will increase and will decrease in galvanic cell 3 (D) The voltage in galvanic cell 3 will increase and will decrease in galvanic cell 1. 22. Consider a voltaic cell based on these half-cells. Ag (aq) +e → Ag/s) Cd (aq) + 2e → Cd¢s) E* = +0.80 V E = -0.40 V Identify the anode and give the voltage of this cell under standard conditions. (A) Ag; Ecell = 0.40 V (B) Ag;…arrow_forwardA reaction is reactant-favored at equilibrium. Will ratio of products to reactants in the cell be the same as equillibrium ?arrow_forward
- A student made measurements on some electrochemical cells and calculated three quantities: • The standard reaction free energy AGº. • The equilibrium constant K at 25.0 °C. • The cell potential under standard conditions E". His results are listed below. Unfortunately, the student may have made some mistakes. Examine his results carefully and tick the box next to the incorrect quantity in each row, if any. Note: If there is a mistake in a row, only one of the three quantities listed is wrong. Also, you may assume the number of significant digits in each quantity is correct. Also note: for each cell, the number n of electrons transferred per redox reaction is 1. calculated quantities (Check the box next to any that are wrong.) cell n aG K E -21 1.42 x 10 A 1 119. kJ/mol O 1.23 V -- В - 51. kJ/mol 1.16 X 10 -0.53 V -11 2.06 X 10 1 -61. kJ/mol 0.63 Varrow_forwardWhich statement about electrolytic cells is false? Electrolytic cells convert electrical energy into chemical energy. O The redox reaction is non-spontaneous. The anode and cathode are placed in the same container with the aqueous electrolyte. O The anode is negative and the cathode is positive. O The external battery supplies the electrons.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY