
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chem help!
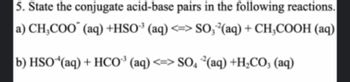
Transcribed Image Text:5. State the conjugate acid-base pairs in the following reactions.
a) CH₂COO (aq) +HSO³(aq) <=> SO, ²(aq) + CH3COOH (aq)
b) HSO (aq) + HCO³(aq) <=> SO4²(aq) +H₂CO3(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A patient is administered a 250 mL IV bag of Vancomycin 1 g. If the bag is to last 2.5 hours, what is the flow rate in mL/hour?arrow_forwardAn infant ibuprofen suspension contains 100 mg/ 5.0mL suspension. The recommended dose is 10 mg/kg body weight. How many milliliters of this suspension should be given to an infant weighing 21 lbs?arrow_forwardhelp me solve this pleasearrow_forward
- An IV pump delivers medication at a contstant rate of 24mg/hr. How long does it take to deliver 90mgarrow_forwardAn intramuscular medication is gicen 5.0 mg/kg of body weight. What is the dose for a 180-lb patient?arrow_forwardUsing any chemical concept in a lab using TLC, make a connection to an event in the news. Your writing should find at least one way, if not more, in which the chemical concept you have chosen is demonstrated.arrow_forward
- Assume the recommended single dose of ibuprofen for children over the age of two is 4.5 mg per pound (mg/lb) of body weight. Use this guideline to calculate a single dose in milligrams for a five-year-old child who weighs 47 lb. dosage: mgarrow_forwardGive “Drug X” 5.0 mg/kg per day in two divided doses. The patient weighs 44 lb.arrow_forwardCalculating BMI with English Units Khloe is 5 feet, 9 inches (5’9”) and 170 lbs. Calculate her BMI. (Round to the nearest 0.1.) what is her weight statusarrow_forward
- 8. Order: Heparin sodium 2000 units subcutaneous in abdomen q12h. Supply: 5000 units/mL. What is the quantity to administer? mL.arrow_forwardMass of sample = 0.2492g Number of insects = 6 Initial watch glass weight = 90.7712g Weighing of watch glass after hexane evaporation = 90.8850g Mass of crude fats = 0.1138g Percent of crude fats in tissue sample = 45.67% 1.) For adults, the recommended daily intake of fats is around 60g. Based on the class results above, how many grams of the tissue analyzed would you have to consume to meet the daily requirement? 2.) How many insects would have to be consumed to meet the daily requirement? 3.) Butter is about 85% fat, and a typical person spreads about 3g of butter on a piece of toast. How many bugs would you have to spread on your toast for an equivalent amount of fat? 4.) The crude fat could also be converted to methylated fatty acids and used as a biofuel in diesel engines and aircraft. Note the mass of the biofuel is essentially the same as that of the crude fats used to make it. a) If the biofuel has a density of 880g/L, how many grams of insects would be needed to make a…arrow_forwardAtropine is given to patients who have dangerously slow heart rates. The initial dosage is 0.5mg/kg. The package for atropine is a 5mL vial that contains 1.0mg/mL. A patient who weighs 264.1 lbs would require how many mL of an initial dose of atropine?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY