
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
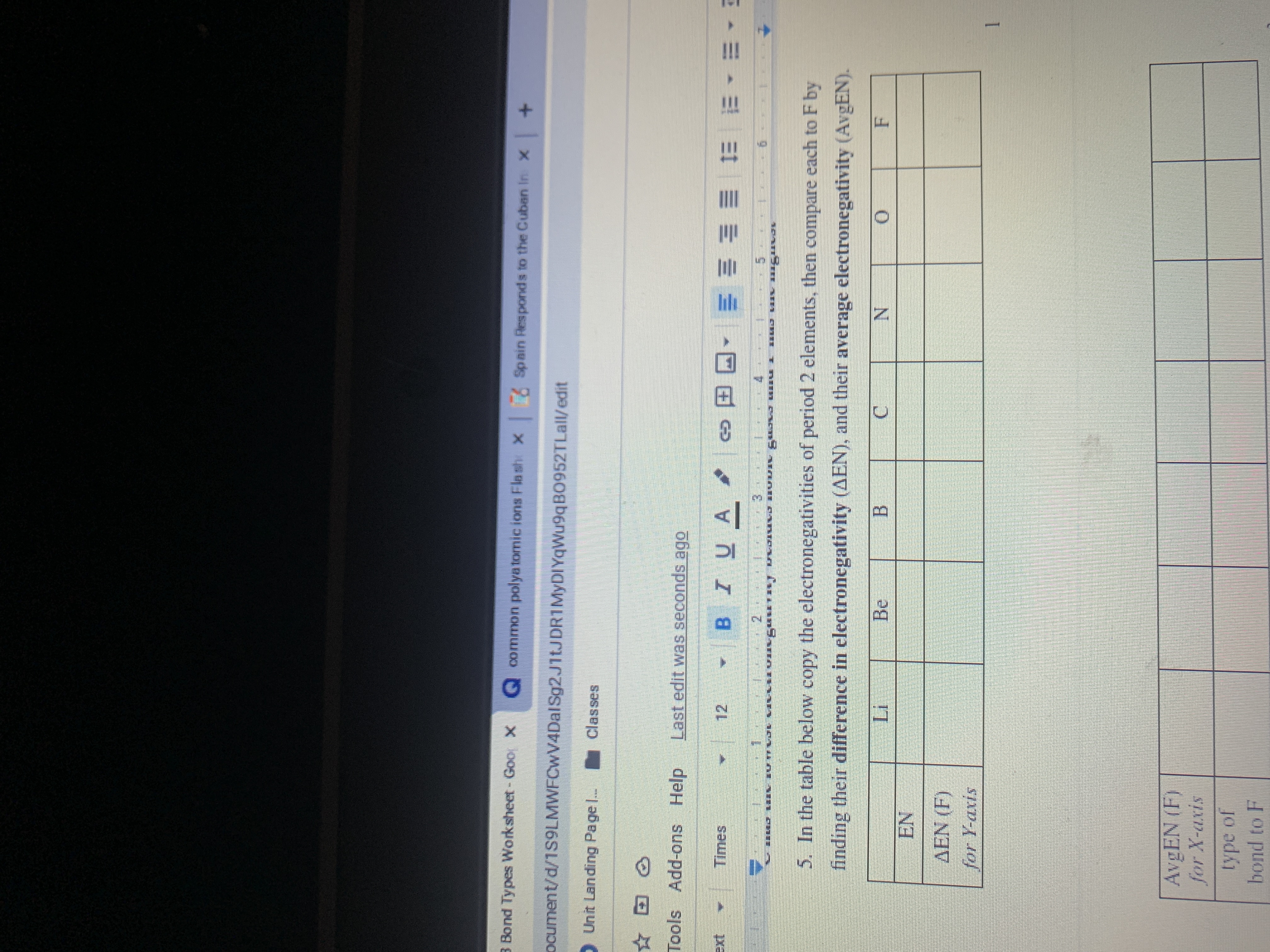
Transcribed Image Text:5. In the table below copy the electronegativities of period 2 elements, then compare each to F by
finding their difference in electronegativity (AEN), and their average electronegativity (AvgEN).
Li
Be
F
EN
AEN (F)
for Y-axis
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Section 3: Worksheet (continued) 4. H;0 |Total number of valence electrons: Lewis structure: 3D sketch: | Molecular shape / bond angle Polar or nonpolar?arrow_forward4. Explain why the following molecules can NOT existis Xarrow_forwardIn the correct Lewis Structure for CH4, how many lor e pairs (unshared pairs) are on the central atom? Give all atoms zero formal charge, C in middle surrounded by H (You can use my easy method for molecules containing C,H,0,N F only if you want) O 1 O 2 O 3 O 4 O 5 O 6 esarrow_forward
- 1. Mark the following statements (A-E) as true or false A. The halogen bond dissociation energy decreases in the row Cl2 Br2 I2 because of repulsion between the electrons in the lower shells. B. The melting points of the dihalogens increase down the group because the interactions between the dihalogen molecules get stronger C. The electronegativity of the halogens decreases down the group because of the decrease of the electron affinity and the increase of the ionization energy. D. The unusually low bond dissociation energy of the F2 molecule is a direct result of the high electronegativity of fluorine E. The high reactivity of fluorine is in part due to the low bond dissociation energy of the F2 moleculearrow_forward101 107 317DOW 3 Draw a Lewis structure for NH 3 in which the central N Atom obeys the octet rulle, and answer the following questions. O waist auto Ch 0 The number of unshared paies (lone pairs) on the centent Natomis: opxo to slam? The central Natoms forms The central N atoms forms double bonds. single bondsin LUE OCULarrow_forward3. Lewis structures of two chemical species are shown below. For each, give (i) the formal charge on each atom and (ii) the overall charge on each species. Structure a Structure b :F :O-CFo H- Atom 1 Atom 2 Atom 3 Atom 4 Atom 5 Хе Oa Ob Fa Fb H Cl Oa Ob Ос రీరేజిిarrow_forward
- In Which of the following Componds would lonic bonds be encountered ? that apply) (Circle or underline all NH 3 PC 3 co2 M gO NH y NO 3arrow_forwardIonizing an H2 molecule to H2+ changes the strength of the bond. Based on thedescription of covalent bonding given previously, do you expect the H¬H bond inH2+ to be weaker or stronger than the H ¬ H bond in H2?arrow_forwarde best Lewis structure for the nitrate ion, NO3. How many single bonds, double bonds, and lone pair of elec entral atom?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY