
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
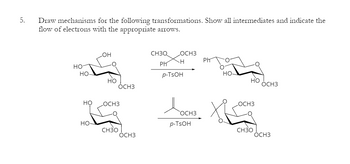
Transcribed Image Text:5.
Draw mechanisms for the following transformations. Show all intermediates and indicate the
flow of electrons with the appropriate arrows.
OH
HO
HO-
но
OCH3
HO
OCH3
CH3Q
OCH3
Ph
H
Ph
p-TSOH
HO-
HO
OCH3
OCH3
Досно Ха
OCH3
HO-
p-TSOH
CH3O
OCH3
CH3O
OCH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Complete the reaction scheme below. Show all reagents and intermediates. No reaction is a possible answer. 1) CH3CH2M9B CH3OH H+ (ехcess) но 2) H30*, H2O B A OCH3arrow_forwardThe transformation below takes place by two distinct reactions. Intermediate A is formed in the first reaction and then this goes on to the product in the second reaction. Provide a complete curved-arrow mechanism for all steps of both reactions.arrow_forwardPlease explain the steps clearer. I tried to get help and there were so many other steps. Please help me understand. Thanksarrow_forward
- Complete the reactions below, draw the mechanism for each reaction, and determine majorand minor products where applicable.arrow_forwardIndicate reagents and conditions needed to impart the transformations below. You do not need to indicate the mechanism, each will require more then one-step.arrow_forwardDraw mechanisms for the following reactions. Use curved arrows to show electron flow for each step and draw out all reactants to effectively show all arrow pushing. Write a written description of the mechanisms.arrow_forward
- Nonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forwardAll rearrangements we have discussed so far have involved generation of an electron-deficient carbon followed by a 1,2-shift of an atom or a group of atoms from an adjacent atom to the electron-deficient carbon. Rearrangements by a 1,2-shift can also occur following the generation of an electron-deficient oxygen. Propose a mechanism for the acid-catalyzed rearrangement of cumene hydroperoxide to phenol and acetone.arrow_forwardPlease show mechanismarrow_forward
- The reaction of methylpropene with HBr in ether gives one of the two products below as the major product. Br HBr Br ether Product A Product B Product would have a higher energy transition state for the formation of the intermediate leading to it. O A O B O Both products would have the same transition state.arrow_forwardComplete the following ether cleavage reaction by moving the correct product into each of the two boxes. ОН HI (ехcess) + heat `CH,OCH3 ОН ОН ОН `CH,I `CH2OH *CH,I CH2I HO, CH3OH CH3I H,O CH;CH,I CH;CH2OHarrow_forward7. Give a Mechanism for the following reaction; show formal charges and significant intermediates. e CH3CH₂NH2 H3O+ N CH₂CH3 + H₂Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning