
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
How do you know which one is the oxidation reaction
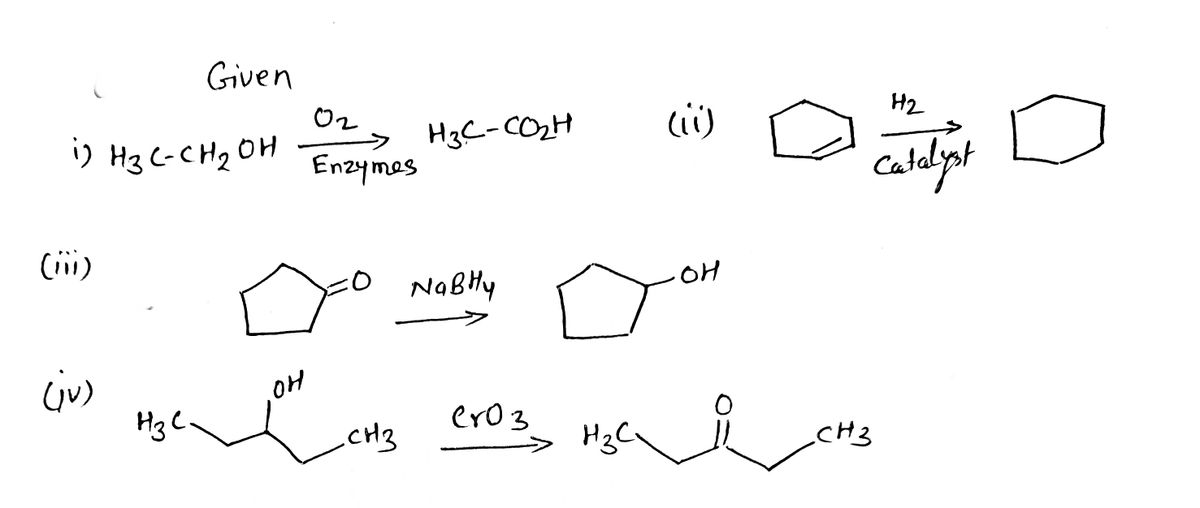
![5. a) From the following chemical reactions identify (circle) the oxidation reactions.
1. Reaction:
\[
\text{CH}_3\text{CH}_2\text{OH} \xrightarrow{\text{O}_2, \text{Enzymes}} \text{CH}_3\text{CO}_2\text{H}
\]
2. Reaction:

\[
\text{Cyclohexene} \xrightarrow{\text{H}_2, \text{Catalyst}} \text{Cyclohexane}
\]
3. Reaction:
\[
\text{Cyclopentanone} \xrightarrow{\text{NaBH}_4} \text{Cyclopentanol}
\]
4. Reaction:
\[
\text{2-Butanol} \xrightarrow{\text{CrO}_3} \text{Butanone}
\]
In these reactions, look for processes involving the gain or loss of oxygen or hydrogen to determine oxidation.](https://content.bartleby.com/qna-images/question/1f8fa2fa-6e6b-4316-8c70-a160edb12e24/4f739c91-00cc-465e-8485-bacf65780bb9/297lkou_thumbnail.jpeg)
Transcribed Image Text:5. a) From the following chemical reactions identify (circle) the oxidation reactions.
1. Reaction:
\[
\text{CH}_3\text{CH}_2\text{OH} \xrightarrow{\text{O}_2, \text{Enzymes}} \text{CH}_3\text{CO}_2\text{H}
\]
2. Reaction:

\[
\text{Cyclohexene} \xrightarrow{\text{H}_2, \text{Catalyst}} \text{Cyclohexane}
\]
3. Reaction:
\[
\text{Cyclopentanone} \xrightarrow{\text{NaBH}_4} \text{Cyclopentanol}
\]
4. Reaction:
\[
\text{2-Butanol} \xrightarrow{\text{CrO}_3} \text{Butanone}
\]
In these reactions, look for processes involving the gain or loss of oxygen or hydrogen to determine oxidation.
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following skeletal oxidation-reduction reaction occurs under basic conditions. Write the balanced REDUCTION half reaction. F- + I2 I- + F2 ____________ -----> ___________ Reactants. Products.arrow_forwardSolid copper(II) chloride is heated strongly in a test tube in a fume hood. identify the substance that is oxidized, and the substance that is reduced.arrow_forwardShow which species is being oxidized and which species is being reduced. Draw the half reactions, showing the changes in oxidation state. How many electrons are being transferred?arrow_forward
- Based on the results of previous oxidation and reduction questions, complete and balance the following oxidation- reduction chemical reaction with proper coefficients in front of each chemical compound. For example, if a coefficient is number 1, put just 1 as a number and do not put anything else before and after. C6H5CHO + C6H5CO₂ + type your answer... type your answer... type your answer... type your answer... MnO4 + H₂O + type your answer... type your answer... OH MnO₂ (s)arrow_forwardThe oxidation of oxgen in the first photo the oxidation of teh ble and red in the seceong photo. with explanation plesearrow_forwardWhich statement is true concerning an oxidation-reduction reaction? O The reactant which is reducedchis the oxidizing reagent. O The reactant which is oxidized is the oxidizing reagent. O The reactant which gains electrons is the reducing agent. O The reactant which loses electrons is the oxidizing agent. O None of the statements above is true. e Textbook and Media Save for Later P Type here to search W IOI 19 24 & 6. 8. Q W Rarrow_forward
- Which of the following statements about oxidation and reduction reactions is true? Group of answer choices None of these are true. Only neutral atoms are formed in oxidation and reduction reactions. More than one electron can be transferred in an oxidation and reduction reaction. A half-reaction is only used for reduction reactions. In a half-reaction, only half of an electron is transferred.arrow_forward11-20arrow_forwardComplete the following oxidation reaction I attached a picture of the problem since I don't know how else to do it. Thank you so much in advance!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY