
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
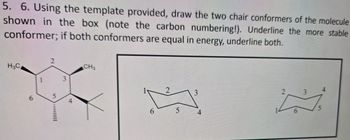
Transcribed Image Text:5. 6. Using the template provided, draw the two chair conformers of the molecule
shown in the box (note the carbon numbering!). Underline the more stable
conformer; if both conformers are equal in energy, underline both.
H3C
6
5
3
4
CH3
2
6
5
4
3
4
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Consider 1-bromo-2-methylpropane and draw the following. (a) The staggered conformation(s) of lowest energy (b) The staggered conformation(s) of highest energyarrow_forwardwhat is the sawhorse projection of the LEAST STABLE ECLIPSED CONFORMATION OF N-PENTANE ROTATING BETWEEN C2-C3?arrow_forwardSketch the chair conformers for the following compound. Then identify which conformer is more and less stable. "CH3arrow_forward
- Draw both chair Conformers of chlorocylohexane. How much higher in energy is the less stable Conformer?arrow_forwardDraw both chair conformations of trans-1,3-dimethylcyclohexane. Label the more stable conformer or write "none". Indicate the presence of any diaxial interactions.arrow_forward2. H3CO H3CO HO 1. Consider the molecule shown below. [1] Draw the two chair conformations for the compound. [2] Identify which of the two chair conformations is more stable and explain why. H3CO Jonoto NH₂ How are the following molecules related? (exactly the same, completely different, constitutional isomers, enantiomers, or diastereomers). OH OH OCH3 HO“ OH H3CO H3CO is content is protected and may not be shared, uploaded of ributed OCH3 HO,,, OH O... "OCH3arrow_forward
- 6. State the relationship between each of the following pairs of structures (same, enantiomers, diastereomers, constitutional isomers or different compound that are not isomeric). b CH3 CH3 **CH3 a CH3 OH OH O d d .& & Br s s C/.. CI Br Да Br Bridarrow_forwardPlease provide a drawing explaning the answer.arrow_forwardFor each of the following pairs of compounds, determine which compound is more stable (may be more helpful to draw out the chair conformations)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning