
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Hello, can I get help on this please? I am confused and do not understand! Thank you!
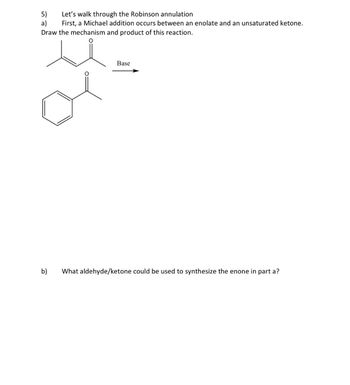
Transcribed Image Text:5)
Let's walk through the Robinson annulation
a)
First, a Michael addition occurs between an enolate and an unsaturated ketone.
Draw the mechanism and product of this reaction.
Base
b)
What aldehyde/ketone could be used to synthesize the enone in part a?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Give me detailed Solution with explanation needed..don't give Handwritten answerarrow_forwardWhat are the products to the reaction depicted below, assuming that NH4* is the acid? NH4* H20 (1) NH,2+ H3O* (aq) NH3 -ОН (аq) NH3 H30* (aq) NH,2+ -ОН (aq) +arrow_forwardHC 0- -CH3 Consider the molecule above. Based on its structural formula, which odor/taste would this substance most likely have? salty/tart bitter/noxious sweet/pleasant sour/tartarrow_forward
- Please don't provide handwriting solutionarrow_forwardClassify each molecule as hydrophilic, hydrophobic, or amphipathic (amphiphilic). بہلا H₂C OH Hydrophilic OH CH3 Hydrophobic Answer Bank CH₂ H OOC-CH₂-C-COO NH3 Amphipathicarrow_forwardClassify the formulas as amines, amides, or neither. Amines HỌC-NH, RCONH, Amides RCH,NH, || CH₂CH₂OCCHCH₂CH₂ CH3 Answer Bank Neither 0 CH, CH, C-NH, COOHarrow_forward
- help mearrow_forwardHow did you know HNO3 is [H+ ] And not [OH-] ?arrow_forwardThe five parts of question 16 relate to the following three molecules: N-H А в с A (a) Which of the three molecules above are structural isomers? (b) Which of the molecules contains a carbon with linear geometry? (c) Which molecule contains a tertiary amine? (d) Which molecule only contains sp³ hybridized atoms (not including hydrogen)? (e) Which molecule is chiral? Circle the stereocentre in this molecule.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY