
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
What is the energy associated with the formation of 1.75 g of 4He by the fusion of 3H and 1H?
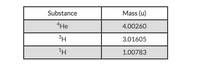
Transcribed Image Text:Substance
Mass (u)
4He
4.00260
3H
3.01605
1.00783
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is spontaneous radiation?arrow_forwardThe COVID-19 pandemic forced many people to work from home for extended periods. During these periods, many cities reported unusually low levels of smog. Suggest an explanation for this observation.arrow_forwardWhat percentage of 149X (t1/2 = 81.9 h) would remain after 37.0 hours. Use the formula with 0.693 instead of ln 2. The data for element X does not exist. All numbers are hypothetical.arrow_forward
- How many moles of methane must be burned to generate the energy produced by fission of 0.560 mol(132 gg) of uranium-235arrow_forwardTwo reactors are connected by a small valve (whose volume can be ignored). The larger one has a volume of 3.00 L and contains F2 at a pressure of 3.000 bar. The small one has a volume of 2.00 L and contains Cl2at a pressure of 2.000 bar. The whole system is kept at 328 °C. When the valve between the chambers is opened, the following reaction occurs: 3 F2(g) + Cl2(g) → 2 ClF3(g) Calculate the mole fractions and partial pressures of all species present after reaction. [Cl2: 14.3% and 0.200 bar; ClF3: 85.7% and 1.20 bar] What is the significance of the coefficients in a balanced chemical equation? How were they used in the question you were assigned?arrow_forwardWhat is the relationship between bond length and pKa?arrow_forward
- If ötzi were indeed a recent corpse, made to look old by the harsh weather conditions found on the high mountain pass, what would you expect the ratio of 14c to 12c to be, relative to that in your own bodyarrow_forwardWhich process would turn Rn-210 into Po-206?arrow_forwardWrite the daughter nucleus product in the following nuclear processes: Beta emission of manganese-56 Gallium-67 decays by electron capture Potassium-38 decays by positron emission.arrow_forward
- If a match contained 0.265 g of P4S3, what would be the energy released in kJ?arrow_forwardWhat is the mass, in megagrams, of 0.3% I would be necessary to form 22271.1L NaIO3 ?arrow_forwardNaturally occurring uranium contains about 0.7% of the fissionable isotope 235U, with almost all the rest 238U. Since gaseous 235UF6 diffuses about 0.4% faster than 235UF6 , diffusion produces a mixture that is slightly enriched in 235UF6 . Multiple diffusion steps – hundreds are needed – can then produce the necessary 235U concentration for use in reactors or bombs. A sample of 235UF6 gas in a 15,000 L cylinder exerts a pressure of 167 kPa. The root-mean-square speed of the 235UF6 molecules is 159 m/s. What is the temperature of the sample of 235UF6 gas? _______Karrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY