
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Hi, I need help with C (H+ in half-neutralized solution), Ka for unknown acid, and part D-change in PH from part C.
Thank you so much!
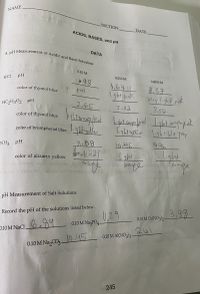
Transcribed Image Text:A. pH Measurement of Acidic and Basic Solutions
NAME
SECTION
DATE
ACIDS, BASES, and pH
DATA
0.10 M
pH
0.010 M
HCI
098
0.0010 M
1,671
2.57
color of thymol blue pink
ght pak
Hay light nat
HC2H3O2 pH
2.65
3.02
3.50
color of thymol blue
color of bromphenol blue htyellar
lightuell Light ble pay
1.00
10.46
JOHT
9.96
NH3 рH
Light
color of alizarin yellow
Oranf
pH Measurement of Salt Solutions
3.89
29
0.10 M N23PO4-
Record the pH of the solutions listed below.
0.10 M Cu(NO32-
261
0.10 M AI(NO3)3.
0.10M Na,0,0,495
245
![LA443 3.79
pH of half-neutralized acid solution
[H*] in half-neutralized solution
Unknown number
K, for unknown acid
13
D. Demonstration of Buffering Action
3,80
pH after addition of NaOH
change in pH from Part C
E. Determination of the Solubility Product of Slightly Soluble Hydroxides
6.93
pH of saturated Mg(OH), solution
uld ional
10.01
pH of saturated Ca(OH), solution
THOUGHT](https://content.bartleby.com/qna-images/question/b1340680-eb9c-48d5-bc71-d4a4fad49dd5/dd5a63eb-48aa-43a4-9297-26d029b331c1/517zvx_thumbnail.jpeg)
Transcribed Image Text:LA443 3.79
pH of half-neutralized acid solution
[H*] in half-neutralized solution
Unknown number
K, for unknown acid
13
D. Demonstration of Buffering Action
3,80
pH after addition of NaOH
change in pH from Part C
E. Determination of the Solubility Product of Slightly Soluble Hydroxides
6.93
pH of saturated Mg(OH), solution
uld ional
10.01
pH of saturated Ca(OH), solution
THOUGHT
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Help me pleasearrow_forward< Which of the following solutions would be expected to have a pH of 7.00? Weak Acid HCN HNO₂ HIO HBrO C6H5COOH Ka 4.9 x 10-1⁰ 4.5 x 10-4 2.3 x 10-¹1 2.5 x 10-⁹ 6.3 x 10-5 Question 4 of 11 Tap here or pull up for additional resources Weak Base HONH, NH3 CoHsNH, H₂NNH₂ C5H5N A) CSIO B) NaF C) LICIO3 D) KNO₂ Submitarrow_forwardCould you send your answer and your working too please?arrow_forward
- Hello, please answer the question. Please show all your work. Thank you.arrow_forward2req #3 w 20 חי E D An aqueous solution contains 0.315 M ammonia. Calculate the pH of the solution after the addition of 1.58x102 moles of nitric acid to 125 mL of this solution. (Assume that the volume does not change upon adding nitric acid). pH = C Submit Answer $ 4 R F Reminders 28 07 de % 5 V Use the References to access important values if needed for this question. Retry Entire Group 5 more group attempts remaining T G 6 B Y Ⓡ H & ve 7 tv U N A * 00 8 J A I M ( 9 K A O ) 0 < o 1 36 7 L W P Email Instructor A Previous LO CA : I V 11 + [ Next Save and Exit O 11 I 1arrow_forwardHE154 S20 Ch17 Sec7-9 Later iCloud Preferences... r Practice 17.12- Enhanced - with Feedback • Part A Find the [OH-] of a 0.50 M pyridine (C5 H5N) solution. (The value of K for pyridine (C5 H,N) is 1.7 x 10-9.) Express your answer to two significant figures and include the appropriate units. НА [ОН ]- Value Units %3D Submit Request Answer v Part B Find the pH of a 0.50 M pyridine (C5 H5N) solution.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY