
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
please answer all 43-45
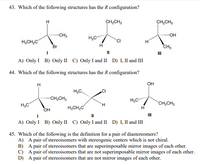
Transcribed Image Text:43. Which of the following structures has the R configuration?
H
CH,CH3
CH2CH3
CH3
он
H,CH2C
CI
Br
CH3
II
A) Only I B) Only II C) Only I and II D) I, II and III
44. Which of the following structures has the R configuration?
H
он
CH¿CH3
H3C
CH,CH3
H.
Он
H,CH2C
II
II
A) Only I B) Only II C) Only I and II D) I, II and III
45. Which of the following is the definition for a pair of diastereomers?
A) A pair of stereoisomers with stereogenic centers which is not chiral.
B) A pair of stereoisomers that are superimposable mirror images of each other.
C) A pair of stereoisomers that are not superimposable mirror images of each other.
D) A pair of stereoisomers that are not mirror images of each other.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The students conducted the assay for LDH activity using the two serum samples. Each cuvette (path length 1 cm) contained 3ml of a suitable assay buffer (including pyruvate as substrate). 20 microlitres of either serum was added to the cuvette and the absorbance values immediately recorded at the optimum wavelength for a period of 5 minutes (absorbance readings taken every 30 seconds). Protein concentration of serum sample (mg/ml) Change in absorbance at optimum wavelength per minute Control serum (C) 8 -0.04 Diseased serum (D) 7.8 -0.6 1c. Using the molar absorption coefficient of NADH as 6220 M-1 cm-1, and by application of the Beer-Lambert law, estimate the enzyme activity in the two samples (C and D). Express activity as moles per second. 1d. Estimate the specific activity of the two samples (moles per second per microgram).arrow_forward30 70- 60- 50- 40- 30 20 10 0₁ 4000 Ророжа Structue nd, C₂H₂1N 3500 иде Sp 3000 2500 18 +2-20 го- 2000 1800 hu 1600 Ф тро 1400 1200 1000 800 6 Н 6 Н 9 Н 600 Уarrow_forward(17360 Systane Part B CH3 CH3 CH3-CH₂-CH-CH-CH₂-OH Spell out the full name of the compound. Submit Request Answer Part C OH CH₂-CH3 P dy 44#1 BAKER -2221 51-19-145 ciation, Inc. OS WINE 19 25 OCT 2022 PM) Address Herbert Epstein 14773 Cumberland C DELRAY BEACH, F အင်းတင်း 46-135654 2 ^ [arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY