
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Please answer part (g) only.
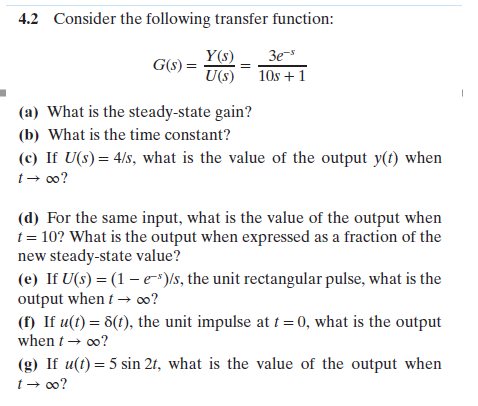
Transcribed Image Text:4.2
Consider the following transfer function:
Y(s) 3e-
U(s) 1Os +1
(a) What is the steady-state gain?
(b) What is the time constant?
(c) If U(s)4/s, what is the value of the output y(t) when
t oo?
(d) For the same input, what is the value of the output when
t 10? What is the output when expressed as a fraction of the
new steady-state value?
(e) If U(s) (1-e-)s, the unit rectangular pulse, what is the
output whent-oo?
( If u() 6(), the unit impulse at 0, what is the output
when-00?
(g) If u(t) 5 sin 2t, what is the value of the output when
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- excel spreadsheetarrow_forwardSummarize your answers in the table provided. Attach a legible and answer sheet upon submission. Points may not be given to any item without a clea unless it is not necessary. Answers with erasures will not be considered. PROBLEM 6.1. Production of Trichloroethylene Trichloroethylene, a widely used degreasing solvent for machine parts, is produced in a two-step reaction sequence. Ethylene is first chlorinated to yield terachloroethane, which is dehydrochlorinated to form trichloroethyle. C₂H4(g) + 2 Cl2(g) → C₂H₂C14(1) + H₂(g) (AH)1=-385.76 kJ/mol C₂H2C14(1)→ C2HCl3(1) + HCl(g) gladins The standard heat of formation of liquid trichloroethylene is -276.2 kJ/mol. Reaction 1: Reaction 2: (W) Enthalpy Values and Standard Heats of Reaction Component AH (kJ/mol) C₂H4(g) Cl₂(g) H₂(g) HCl(g) C₂H₂C14(1) C₂HCl3 (1) Q (kW) Reaction Reaction 1 Reaction 2 (VD) TUO Hn lato T (WA) veisdial (lom\34) H dom) a Assuming W, APE, and AKE are negligible, determine the amount of heat (Q) evolved if 300 mol/h…arrow_forwardUsing the data below, construct a calibration curve. Include the error bars and the equation of the line and the linearity (R2) of the curve. Calcium concentration (ppm) (X-data) Absorbance (Y-data) Run 1 Absorbance (Y-data) Run 2 Absorbance (Y-data) Run 3 0.3 1109 1069 1155 1.2 1225 1168 1233 2.1 1472 1319 1389 4.2 1497 1523 1472 8.0 1833 1898 1849 16 2066 2012 2051arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The