
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
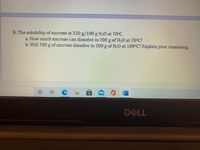
Transcribed Image Text:**Educational Content: Solubility of Sucrose**
### Problem 4:
The solubility of sucrose is **320 g/100 g H₂O** at **70°C**.
a. **Question**: How much sucrose can dissolve in 200 g of H₂O at 70°C?
**Answer**: Given the solubility, you can calculate the amount of sucrose that can dissolve in 200 g of water by setting up a ratio. Since 320 g of sucrose dissolve in 100 g of water, in 200 g of water, twice the amount can dissolve, which is 640 g of sucrose.
b. **Question**: Will 700 g of sucrose dissolve in 200 g of H₂O at 100°C? Explain your reasoning.
**Answer**: At 100°C, the solubility of sucrose typically increases, meaning more sucrose can dissolve compared to 70°C. However, without specific data for 100°C, a definitive answer requires the actual solubility value at 100°C. Generally, with the assumption that solubility increases, it is likely that 700 g could dissolve at this higher temperature.
Note: For accurate results, consult solubility tables specific to 100°C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A. Calculate the freezing point of a solution containing 11.4 g FeCl3 in 180 g water. Calculate the boiling point of the solution. B. Calculate the freezing point of a solution containing 4.7 % KCl by mass (in water). Express your answer using two significant figures.Calculate the boiling point of the solution. C. Calculate the freezing point of a solution containing 0.160 mMgF2.Calculate the boiling point of the solution above.arrow_forwardChoose the aquaeous solution with the lowest freezing point. All solutions are nonvolatile. a. 0.200 m Ca(CLO4)2 B. 0.200 m C6H12O5 c. 0.200 m Al2(SO4)3 d. 0.200 m K3PO4 e. they all have same freezing pointarrow_forwardhow is the solubility of a solid in a liquid affected by temperature?arrow_forward
- 1. A solution of potassium chloride, KCI, has 20 grams of the salt dissolved in 100 grams of water at 40 °C. Approximately how many more grams of the salt can be added to the solution before reaching the saturation point? 2. Determine the molarity of saturated Nacl (Na=23, Cl=35.45) solution at 90 c. 100 90 80 NaNO 70 60 CaCl 50 Pb(NO3)2 40 KCI NaCl 30 20 KCIO, 10 10 20 30 40 50 60 70 80 90 100 Temperature (°C) Solubility (g of salt in 100 g H,0) SONY K,Cr,O,arrow_forward46.29 g of KOH is dissolved in 250. mL of water. (Please pay attention to sig figs!) What is the mass percent of KOH in this solution? (use the density of water 1.00g/mL) a. b. What is the concentration, expressed in molal (m = moles solute/kg solvent), of this initial solution? What is the concentration, expressed in molar (M = moles solute/liters solvent), of this initial solution? (assume that the addition of the KOH to the water did not change the C. volume, which is still 250.mL) d. If I add 500.mL of pure water to this 250.mL solution, what is the new concentration expressed in molar (M)? (Careful, what is the new volume after mixing?) e. If, instead, I took the 250. mL solution and evaporated the water until the solution had a volume of 150.mL, what would be the concentration of this this new solution expressed in molar (M)?arrow_forwardCalculate the solubility of CaF, in water at 25 °C. You'Il find K, data in the ALEKS Data tab. s. Round your answer to 2 significant digits. g x10arrow_forward
- 11. You have a solution that is 18.2% sodium thiosulfate, Na2S2O3 , by mass. a. What mass of sodium thiosulfate is in 75.0 g of solution? b. How many moles of sodium thiosulfate are in 75.0 g of solution? please show workarrow_forwardExplain the difference between a. a colloidal and a crystalline precipitate b. precipitation and coprecipitation c. peptization and coagulation of a colloid d. occlusion and mixed-crystal formation e. nucleation and particle growtharrow_forwardA solid mixture consists of 25.5 g of KNO3 (potassium nitrate) and 4.5 g of K₂SO4 (potassium sulfate). The mixture is added to 130. g of water. Use this solubility curve (Figure 1) to answer the Part C If the solution described in the introduction is cooled to 0 °C what mass of K2SO4 will crystallize?arrow_forward
- Helparrow_forwardAn aqueous solution at 28°C contains 5.6 g of a protein in a 325 mL sample. The osmotic pressure is 0.0313 atm. What is the molar mass of the protein? Show your work in a separate sheet. a. 1.36 × 104 g/mol b. 1.27 × 103 g/mol c. 2.43 × 103 g/mol d. 63.1 g/mol e. 6.94 × 102 g/molarrow_forwardCalculating the freezing point of an aqueous solution 3. What is the freezing point of an aqueous solution that contains 3 moles of the ionic solute Cack per once kilogram of water? €a* + 2CI Cacl2 4. What is the freezing point of an aqueous solution that contains 2.30 moles of rock salt (NaCI) per one kilogram of water? 5. What is the freezing point of an aqueous solution that contains 1.3 moles of MgC12 per 1 kg water?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY