
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
4. Which molecule has the lowest pKa for the H proton at the very bottom (the H that's in the same spot for all the molecules)? Please explain your reasoning.
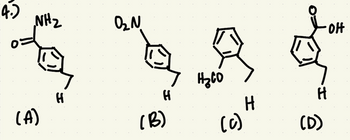
Transcribed Image Text:NH₂
(A)
H
0₂ N
H
(B)
H₂Co
(c)
H
H
(D)
OH
Expert Solution
arrow_forward
Step 1: concept
Based on the stability of conjugate base of compound, acidity vary. With increasing the stability of conjugate base, acidity increases.
+R group increase the electron density on benzene ring that results decrease in acidity.
-R group decrease the electron density on benzene ring that results increase in acidity.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which nitrogen atom in the following molecule cannot be protonated in the presence of an acid? N₂ Select one: O a. Nitrogen a o b. Nitrogen b O c. Nitrogen c O d. Nitrogen d O e. Nitrogen e o f. All nitrogen atoms can be protonated in the presence of an acid. O g. All nitrogen atoms cannot be protonated in the presence of an acid.arrow_forwardI think answer is C... explanation?arrow_forwardLabel all of the acidic hydrogen. (Do not count any of the ones that are not shown) bns 2001320 Circle the one that is the most acidic kne noltamoino siano ws Explain, using CARDIO, why it is the most acidic. H H H H N. H. H H stasib was baie vincul CI 6057 enim09 ogmo) ons nod:6) istim relev H S WH bus il basi WH Uns 2506 A toarrow_forward
- 1. For each set, rank the acids by putting a "1" under the weakest acid (highest--most positive-- expected pka), then a "2" under the next most weakest, then a "3" under the next most weakest then a "4" under the strongest acid (highest--most positive-- expected pKa) briefly explain why. H₂ Te a. H₂Se b. HC1 C. H₂S H₂S MOM OH H₂O SIH4 PH 3 OH -Ō OH OHarrow_forwarda If an acid, HA, dissolves in water such that the Ka is 70000, what is the pK, of that acid? a pK. : aarrow_forwardRank the acids in the table below from strongest (1) to weakest (4). The most acidic H atom in each acd has been highlighted. H. H. H (Choose one) (Choose one) (Choose one) (Choose one)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY