Question
complete these following
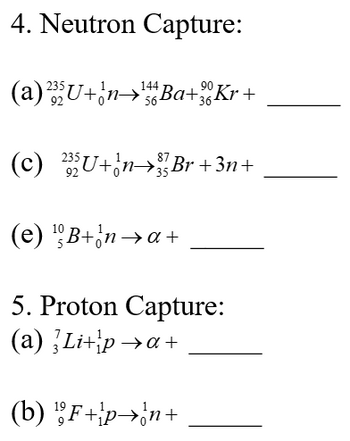
Transcribed Image Text:4. Neutron Capture:
(a)
87
(c) ²35U+¦n→³Br +3n+
(e) ¹B+na+
5. Proton Capture:
(a) Li+pa+
(b) ¹F+p→n+
144
²35U+n→Ba+Kr+
56-
92
90
36-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Similar questions
- What becomes of the loss in mass when heavy nuclei split? b) What formula can be used to account for this loss?arrow_forwardCompute the approximate nuclear radius of: (₂) (c) Palladium-46arrow_forwardSuppose that a nucleus captures two neutrons and decays to produce one neutron. Is this process likely to produce a chain reaction? Explain your response (make sure to proofread and properly give credit to any sources used)arrow_forward
arrow_back_ios
arrow_forward_ios