
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
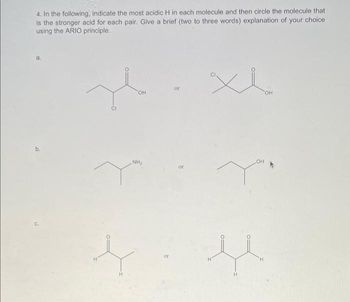
Transcribed Image Text:4. In the following, indicate the most acidic H in each molecule and then circle the molecule that
is the stronger acid for each pair. Give a brief (two to three words) explanation of your choice
using the ARIO principle.
a.
x
or
OH
NH₂
or
or
OH
H
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the Brønsted theory, classify the compounds as either an acid or a base. C,H,OH CH,CH,O Acid CH,CH,NH Answer Bank Basearrow_forward6arrow_forwardFinding the conjugate of an acid or base Fill in the missing chemical formulae in the tables below. acid conjugate base base conjugate acid 2- H,0 co H CI NH, + NH4 H,PO4 Explanation Chocl,arrow_forward
- Write the formula for the conjugate acid of each base. 1. NH3 2. ClO4− 3. HSO4− 4. CO32−arrow_forwardWhat is the K, of cinnamic acid if a 0.040 M cinnamic acid aqueous solution has a pH of 2.93? O 3.4x10-2 O 1.19×10-3 O 4.44 O 3.6x10-5arrow_forwardFor the following reactions, label each species as an acid or a base. Indicate the species that are conjugates of one another. a H2PO42 1 HCO32 ∆ HPO422 1 H2CO3 b F−+HSO4−mHF+SO42− c HSO4− + H2O m SO42− + H3O+ d H2S 1 CN2 ∆ HS21HCN Question 15.36 part 2 select the base Question 15.36 part 3 select conjugate acid Question 15.36 part 3 select conjugate basearrow_forward
- Fill in the missing chemical formulae in the tables below. acid HCO, NHA H₂SO4 conjugate base 0 0 0 base H₂O 2 so² 4 Br conjugate acid 0 × Śarrow_forwardNote to the medically inclined: Uric acid crystals accumulate in joints of people who suffer from gout. The most common joint affected is the big toe. Based on the structure you see below, derive a Lewis representation for the most stable conjugate base of uric acid. :0: H H C' -N-Harrow_forwardBetween HNO3 or HNO2 which is the stronger acid? Type in formula exactly.arrow_forward
- Please help with number 4arrow_forwardComplete the equation for the ionization of water by drawing the conjugate acid and conjugate base. Include lone pairs of electrons. .. H-O H + —O H— ∙H conjugate acid + 1 + [ Í conjugate basearrow_forwardComplete the balanced equation for HC₂H3O₂ dissolved into water. Label the Acid, Base, Conjugate Acid, and Conjugate Base. HC₂H3O2 + H₂O C₂H302-1 + a. acetic b. hydrochloric c. phosphoric e. hydrofluoric f. sulfuric g. sulfurous h. hydrosulfuric 1. Mg+2 -1 n. HPO4² 0. H₂PO4-¹ p. CO3-² u. H2O v. H₂CO3 W. PO4-³ aa. conjugate acid bb. base d. perchloric i. OH-1 j. H30+1 K. SO4² q. HCO3-1 r. H₂S x. HC₂H302 y. C₂H30₂-1 cc. conjugate base S. HS-1 z. acid t. S-2 m. Cl-1arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY