
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
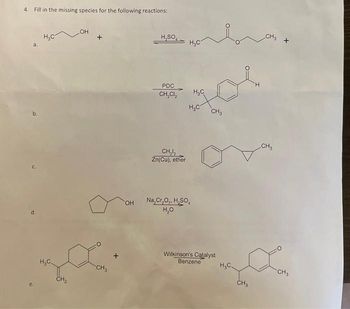
Transcribed Image Text:4. Fill in the missing species for the following reactions:
a.
b.
d.
H₂C
H₂C
CH₂
.OH
CH₂
OH
H₂SO
PDC
CH₂Cl₂
CH₂₂
Zn(Cu), ether
H₂C
H₂C
H₂C
Na₂Cr₂O₂, H₂SO,
H₂O
las+
CH3
CH3
Wilkinson's Catalyst
Benzene
H₂C
CH₂
H
CH3
CH₂
MULTICY
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the products, intermediates, and/or reagents for the following reactions:arrow_forward31. A sample of meat scrap weighing 2.000 g is digested with concentrated H2SO4 and a catalyat. The resulting solution is made alkaline with NaOH and the liberated ammonia distilled into a 50.0 mL of 0.6700 N H2SO4. The excess then requires 30.10 mL of 0.06520 N NaOH for neutralization. What is the percentage of nitrogen in the meat?arrow_forwardWhat is Kc for the reaction: N22CO3 (s) + CO2 (g) + H2O (g) =2 NaHCO3 (s)? [NAHCO3]^2 / [Na2CO3] x [CO2] x [H2O] O [co2] x [H20] O 1/ [CO2] x [H2O] 2 x [NAHCO3] / [Na2CO3] x [CO2] x [H20]arrow_forward
- A + Br₂ CH3OH 1.03 2. DMS KMnO4, NaOH cold HI mCPBA 1. OSO4 2. H₂O2 1.03 2. (CH3)2S 17 18 16 15 19 14 CH3CO3H (peroxyacid) B 13 H₂ Pd or Pt (catalyst) HBr 20 1 1. МСРВА 2. H3O+ 12 11 10 2 Pd or Ni (catalyst) D2 (deuterium) 3 4 LO 5 6 7 8 EtOH Br₂ C BH 3 THF H OH H chu chon d ""H OH "OH a H HBr ROOR (peroxide) H₂O H₂SO4 HCI 'OH 1. BH 3 THF 2. H₂O₂, NaOH Br₂ Br₂ H₂O D + H OH a ď™ œ™ OH H ""H OH H HO"arrow_forwardWhat are the products of the following reactions?arrow_forwardWhen a mixture containing cations of Analytical Groups I–III is treated with H 2S in acidic solution, which cations are expected to precipitate? a. Analytical Group I only b. Analytical Group II only c. Analytical Groups II and III d. Analytical Group III only e. Analytical Groups I and IIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
