
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
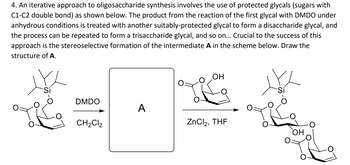
Transcribed Image Text:4. An iterative approach to oligosaccharide synthesis involves the use of protected glycals (sugars with
C1-C2 double bond) as shown below. The product from the reaction of the first glycal with DMDO under
anhydrous conditions is treated with another suitably-protected glycal to form a disaccharide glycal, and
the process can be repeated to form a trisaccharide glycal, and so on... Crucial to the success of this
approach is the stereoselective formation of the intermediate A in the scheme below. Draw the
structure of A.
DMDO
CH₂Cl2
A
OH
ZnCl₂, THF
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following best describes the disaccharide shown here? HO OH OH HO OH OH AmoH HO A. a-1,4' glycoside of D-glucose and D-galactose B. B-1,3' glycoside of D-glucose and D-galactose C. a-1,4' glycoside of D-galactose and D-glucose D. B-1,4' glycoside of D-gulose and D-galactose E. a-1,3' glycoside of D-allose and D-glucose F. a-1,3' glycoside of D-altrose and D-galactose G. a-1,3' glycoside of D-idose and D-idose H. B-1,3' glycoside of D-altrose and D-galactose O A OB OC OD OE OF OG OH F3 1-0 F4 FS MacBook Air FBarrow_forwardCompare the following monosaccharides and choose the best description. a. same sugar b. enantiomers c. diastereomers d. different sugarsarrow_forwardSelect the glycoside(s) that are formed when the following monosaccharide is treated with CH3CH₂OH, HCI. HO OH OH HO- НО. OH OCH₂CH₂ OH OH OH OCH₂CH₂ OH OH OH -OH HO OH OH OH С HO- OH CH3CH₂OH HCI OH OH OCH₂CH3 -OCH₂CH3 a НО НО OH OH OH OH OH OCH₂CH₂ OCH₂CH₂ Lo -OH HO OCH CH НО. OH OH -0 OH OCH₂CH₂ OH -OH OCH₂CH₂arrow_forward
- A disaccharide is shown below. For part (a), circle the anomeric carbon atoms. For part (b), explain whether it is a reducing sugar or non-reducing sugar.arrow_forwardConsider N-acetyl-d-glucosamine Q.) Draw a chair conformation for the disaccharide formed by joining two units of the pyranose form of N-acetyl-d-glucosamine by a b-1,4-glycosidic bond. If you draw this correctly, you have the structural formula for the repeating dimer of chitin, the structural polysaccharide component of the shell of lobsters and other crustaceans.arrow_forwardA Fischer projection of a monosaccharide is shown below (see photo): classify this monosaccharide (e.g., aldotriose) does it have the D or L configuration?arrow_forward
- Provide the systematic name of the cyclic monosaccharide shown here Be sure to include the structural form as a component of the name. H Question 78 of 95 OH CH₂OH H OH H mann H OH O B- D- a- L- fruct gluc pyran furan galact ose oside ol OH Harrow_forwardCompare the following monosaccharides and choose the best description. a. different sugars b. same sugar c. enantiomers d. diastereomersarrow_forwardA) Define "reducing sugar." (b) Sucrose is a disaccharide composed of glucose and fructose (Glc(1 → 2)Fru). Explain why sucrose is not a reducing sugar, even though both glucose and fructose are.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY