
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
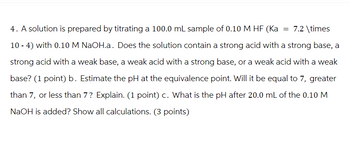
Transcribed Image Text:4. A solution is prepared by titrating a 100.0 mL sample of 0.10 M HF (Ka = 7.2 \times
10-4) with 0.10 M NaOH.a. Does the solution contain a strong acid with a strong base, a
strong acid with a weak base, a weak acid with a strong base, or a weak acid with a weak
base? (1 point) b. Estimate the pH at the equivalence point. Will it be equal to 7, greater
than 7, or less than 7? Explain. (1 point) c. What is the pH after 20.0 mL of the 0.10 M
NaOH is added? Show all calculations. (3 points)
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Consider a buffer made by adding 132.8 g of NaC-H5O, to 300.0 mL of 1.97 M HC-H.O, (Ka = 6.3 x 10-5) a. What is the pH of this buffer? b. What is the pH of the buffer after 0.750 mol of H+ has been added? c. What is the pH of the buffer after 0.250 mol of OH- has been added?arrow_forwardA student added 501 mL of 0.35 M HCl to a buffer solution made up of 0.500 mol of citrate (A-), 0.500 mol of citric acid to 1.5 L of water (total volume = 1.5 L) (Ka = 7.41 x 10-4). A.) Calculate the pH of the initial buffer solution (before the addition of the acid). B.) Calculate the pH of the solution after the addition of HCl referenced above. C.) Did the buffer solution exceed its buffer capacity?arrow_forwardAnswer the following questions about a titration between 20.0 mL of a weak base (Kb= 4.7*10-6) and 0.150 M hydrochloric acid. A.What is the concentration of the weak base solution if it requires 26.67 mL of the HCl solution to reach the equivalence point (assume 1:1 stoichiometry)? B.What is the initial pH of the weak base solution before any HCl is added? C.What is the pH of the titration at the equivalence point? D.The pKa of bruhmophenol is ~ 4.9 and the pKa of thymol blue ~ 9.2. Which indicator would be best to use in the titrationarrow_forward
- A buffer is prepared by mixing 25 mL of 0.10 M nitrous acid (HNO2) and 25 mL of 0.15 M sodium nitrite (NaNO2). The Ka for nitrous acid is 5.6 x 10-4. a.) What is the pH of the buffer solution? b.) What is the pH change when 5 mL of 0.10 M HCl is added to the solution?arrow_forwardanswer plsarrow_forward5. Calculate the pH of a Buffer of Lactic Acid (HC2H502) 0.12M and Potassium Lactate (KC2H502) 0.11M. The Ka = 1.4x10-4arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY