
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:4) Write the balanced chemical equation for the complete
combustion reaction of C,H,
02 (g)
Energy
CO2 (g)
СЗН6 (g) +
H2O (g)
+
5) Draw the condensed structural formulas for all
carbonyl-containing structural isomers of C,H,OBr.
6) Draw the line-angle structural formula for the product of
the following reaction:
CH3
H+
I+ H-OH
|+ H-ОН
7) Draw the line-angle structural formula(s) for the product(s)
of the following reaction:
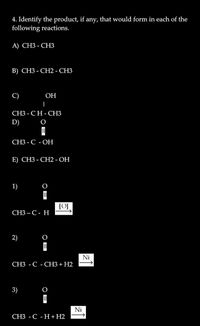
Transcribed Image Text:4. Identify the product, if.
following reactions.
any,
that would form in each of the
А) СНЗ - СНЗ
В) СНЗ - СН2 - СНЗ
C)
ОН
|
СНЗ - СН-СНЗ
D)
СЗ - С -ОН
E) СНЗ - СН2 - ОН
1)
СНЗ— С- Н
2)
Ni
СЗ - С - СНЗ + Н2
3)
Ni
СЗ - С -Н + Н2
OB
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Lead (Pb) has been used for centuries. The Romans used it in their plumbing systems - the symbol for lead (Pb plumbus). To extract the metal, lead (II) sulfide (mineral galena) is roasted in air to form lead (II) oxide (PbO): 2 PbS(s) + 3 O2(g) → 2 PbO(s) + 2 SO2(g) AHrxn = -827.4 kJ Then the lead (II) oxide is reduced with carbon to the metal lead: PbO(s) + C(s) Pb(s) + СО (9) AHrxn = +106.8 kJ a) Use the equations above and Hess's Law to determine the enthalpy of change for the following reaction: PbS(s) + /2 O2(g) + C(s) → Pb(s) + CO (g) SO2(g) ΔΗη [ Select ] kJ (1 mol PbS) b) How much energy (kJ) absorbed (+AHxn) or evolved (-AHpxn) when 400.0 grams of lead (II) sulfide (PbS(s) is converted to lead (Pb)? ΔΗΡη [ Select ] kJ / 400.0 g PbSarrow_forwardConsider the reaction for the production of NO₂ from NO: 2 NO(g) + O₂ (g) → 2 NO₂ (g)arrow_forwardConsider the reaction involving the burning of sulfur: 2S(s) + 3O2(g) ->> 2SO3(g) delta Hrxn= ? ? ?arrow_forward
- Which of the following is the formation reaction for Na2CO3 (s)? A. 2 Na(s) + C(s, graphite) + 3 O(g) → Na2CO3(s) B. 4 Na(s) + 2 C(s, graphite) + 3 O2(g) → 2 Na2CO3(s) C. 2 Na(s) + C(s, graphite) + 1.5 O2(g) → Na2CO3(s) D. Na2(s) + C(s, graphite) + 1.5 O2(g) → Na2CO3(s) E. 2 Na(g) + C(g) + 1.5 O2(g) → Na2CO3(s)arrow_forwardUse the References to access important values if needed for this question. When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: NH3 (g) + 02 (g) NO (g) + H2O (g)arrow_forward4. Imagine that you are given 0.2500 g of a sample of copper(II) sulfate pentahydrate (CuSO4 • 5 H2O). You very carefully heat the compound for an extended period of time to drive off water, after which you determine the mass of the remaining solid to be 0.1598 g. (i) Determine whether the data given confirm the formula of the hydrate. You must (ii) show any relevant calculations that support your answer.arrow_forward
- Balance the chemical equation for the combustion of ethanol _CH3CH2OH(g) +_02(g)→CO2(g) +_H2O() _CO2(g) +_H20(1) How many grams of oxygen gas are required to burn 1.4375 g of ethanol?arrow_forwardgiven the following equation 2 Co2O3(s) + 3 C(s) --> 4 Co(s) + 3 CO2(g) indicate the coefficient for elemental carbon 6 4 1 3 or 2arrow_forwardThe following chemical equation is not balanced. What must be done to the equation to make it balanced? Al (s) + CuO (s) – Al203 (s) + Cu (s) Oa) Change the coefficients to: 2 for AI, 3 for CuO, and 3 for Cu. b) Change the subscript of CuO to CuO3. C) Add a molecule of AIO. d) Change the state of matter in the reactants. O e) Change the subscripts of Al,03 to be AIO.arrow_forward
- 6H2O + 6CO2 → 1C6H12O6 + 6O2 In photosynthesis, water reacts with carbon dioxide to give oxygen and glucose (C6H12O6). How many moles of CO2 are required to make 120.0g of glucose?arrow_forwardBalance the following chemical equation and determine the number of moles of Fe(s) produced when 1 moles of Fe2O3 (s) react with excess C(s) to produce Fe(s) and CO₂ (g). Fe2O3(s) + C(s) → Fe(s) + CO₂(g)arrow_forward3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY