
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

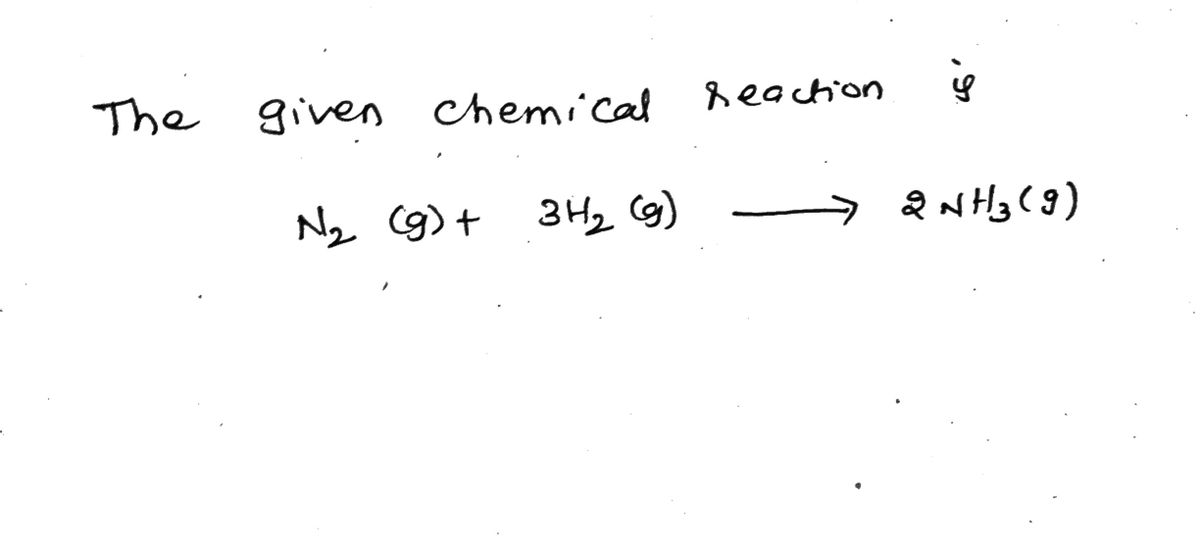
Transcribed Image Text:Use the data found in the Thermodynamic Values link under Reference Materials to calculate AH° and AS° at 25 °C for
N2 (g) + 3 H₂ (g)
→ 2 NH3(g)
Although the AH° and AS° values do change slightly with temperature, assume that the change is not large enough to significantly affect
the outcome of the following calculation. Use the AH° and AS° values to determine the temperature (in K) at which this reaction will
become nonspontaneous.
Enter a number - no units.
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the gas phase reaction NO + O2 NO2 for which ΔH° = –57.05 kJ and K = 1.54 × 106 at 25°C. Calculate Δ G° at 25°C for the following reaction: 2NO + O 2 2NO 2 a. –5.92 kJ b. 70.6 kJ c. –35.3 kJ d. 5.92 kJ e. –70.6 kJarrow_forwardAt 455 K, AG° = -21.1 kJ/mol for the reaction A (g) + 2 B (g)→3 C (g). If the partial pressures of A, B, and C are 1.21 atm, 3.25 atm, and 0.235 atm respectively, what is the free energy for this reaction? %3Darrow_forwardConsider the reaction 2N₂(g) + O₂(g)- →2N₂O(g) Use the standard thermodynamic data in the tables linked above. Calculate AG for this reaction at 298.15K if the pressure of N₂O(g) is reduced to 11.52 mm Hg, while the pressures of N₂(g) and O₂(g) remain at 1 atm. ANSWER: kJ/molarrow_forward
- Mg(s) + 2HCI(aq) MgCl, (aq) + H2 (g) + 1550kJ For this reaction at 25°C, Change in enthalpy: [ Select ] O because [ Select] • Change in entropy: [ Select ] O because [ Select ] · Change in Gibbs free energy: [ Select] thus • This reaction is [ Select ]arrow_forwardConsider the reaction: NH3 (g) + HCI (g) → NH4Cl (s) Given the following table of thermodynamic data at 298 K: Substance AH° (kJ/mol) S° (J/K-mol) NH3 (g) -46.19 192.5 HCI (g) -92.30 186.69 NH4Cl (s) -314.4 94.6 The value of K for the reaction at 25 °C is 1.1-10-16 9.3 - 1015 1.4.108 150 8.4. 104 Submit Request Answerarrow_forwardUse the standard free energy of formation data to determine the free energy change for each of the following reactions, which are run under standard state conditions and 25 °C. Identify each as either spontaneous or nonspontaneous at these conditions. a. MnO₂ (s) → Mn (s) + O₂ (g) b. H₂(g) + Br2 (1)→ 2HBr (g) c. Cu (s) + S (g) → CuS (s) d. 2LiOH (s) + CO₂ (g) → Li₂CO3 (s) + H₂O (g)arrow_forward
- Delta H degrees and Delta S degrees for a reaction are –11.7 kJ/mol and –105 J/mol K, respectively. At what temperatures (in K) will this reaction be spontaneous under standard conditions?arrow_forwardPlease don't provide handwritten solution ....arrow_forward10) Use the Standard Free Energy of Formation values below to calculate Standard Free Energy for the balanced equation: PbCl₂(s) + 2NOF(g) →→→ PbF₂(s) + 2NOCl(g) Substance AGran (kJ/mol) PbCl₂(s) -314.2 NOF(g) -50.29 PbF₂(s) -630.9 NOCI(g) 66.07 Scanned with CamScannerarrow_forward
- Dissolution of ionic compounds generally involves an increase in entropy (+ΔS°), but for a few specific metal ions, dissolution of the ionic compound can lead to a decrease in entropy. For example, the dissolution of magnesium chloride (MgCl2) has a ΔS° = -115 J K-1 mol-1. Explain this decrease in entropyarrow_forwardAt 1120 K, AG° = 89.1 kJ/mol for the reaction 3 A (g) + B (g) →2 C (g). If the partial pressures of A, B, and C are 11.5 atm, 8.60 atm, and 0.510 atm respectively, what is the free energy for this reaction? %3Darrow_forwardIn the reaction N2O4 = 2NO2, it is given that the AH298 and AS298 are +55.3kJ and +176J/K, respectively. Calculate AG at room temperature and discuss the spontaneity of this reaction at different temperature ranges.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY