
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
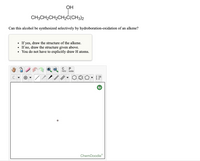
Transcribed Image Text:ОН
CH3CH2CH2CH2Č(CH3)2
Can this alcohol be synthesized selectively by hydroboration-oxidation of an alkene?
If yes, draw the structure of the alkene.
If no, draw the structure given above.
You do not have to explicitly draw H atoms.
P
opy aste
ChemDoodle
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can I get help with this pleasearrow_forwardMatch the descriptions to the structures. AHA a tertiary Alcohol ACA an epoxide ATA a symmetrical ether a non-oxidizable alcohol a secondary alkyl halide ६.१ ADA AJA 2 HOOC AEA AKA OH dor AFA ů ALA tom AGA AMAarrow_forwardAlcohols can be converted to alkyl bromides using PBr3, which causes a complete inversion of stereochemistry. OH 10 PBr 3 Draw the stepwise mechanism for bromination of an alcohol. Be sure to include non-zero formal charges and lone pairs as necessary. : OH Br of 0 Br. Br Br Add/Remove step X Click and drag to st= drawing a structurarrow_forward
- Give the organic product. H :0. + NO: NO₂ HNO; H₂SO 2 NO: NO₂arrow_forwardPredict the product of this organic reaction: OH CH3-C-CH3 CH3 [0] P + H₂O Specifically, in the drawing area below draw the condensed structure of P. If there isn't any P because this reaction won't happen, check the No reaction box under the drawing area. Note for advanced students: you can assume the reaction is run under mild conditions. Click anywhere to draw the first atom of your structure. X Ö Śarrow_forwardGive clear handwritten answer of all subparts please!arrow_forward
- When 3.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere, the reaction shown below occurs and the temperature of the resulting solution rises from 22.00°C to 47.40°C. If the specific heat of the solution is 4.18 J/(g ⋅ °C), calculate ΔH for the reaction, as written. Ba(s) + 2 H2O(l) → Ba(OH)2(aq) + H2(g) ΔH = ?arrow_forwardDraw the product of the following reaction H*, heat + H2O Circle and name the functional groups in this molecule Но HN, OHarrow_forwardDraw a structural formula for the major organic product of the following reaction: -CH=CH₂ + Br₂ ***** • Show product stereochemistry IF the reactant alkene has both carbons of the double bond within a ring. • Do not show stereochemistry in other cases. • If enantiomers are formed, just draw one. Sn [1 CH₂Cl₂ ChemDoodle Previous Nextarrow_forward
- 2. YATEMBRO MADRO JAREMBO BESMHO OH Start from acetylene (HC=CH) to make this alcohol. Use any other appropriate organic or inorganic reagents.arrow_forwardCh19. Aldehydes and Ketones: Nucleophilic Addition Reactions Nucleophilic Addition Reaction Aldehydes and ketones are reduced to yield 1° and 2° alcohols, respectively - Reduction (hydride attack: H): NaBH, or LIAIH, (stronger) _H 1) NaBH4 2) HⓇ - Grignard reagents (R-MgX) give alcohols MgBr 1) + - Addition of HCN yields cyanohydrins, RCH(OH)CEN NaCN 2) H₂0* HCNarrow_forwardDraw the major organic product(s) for the following reactionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY