
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
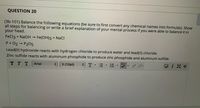
Transcribed Image Text:QUESTION 20
(3b-101) Balance the following equations (be sure to first convert any chemical names into formulas). Show
all steps for balancing or write a brief explanation of your mental process if you were able to balance it in
your head.
FeCl3 + NaOH →
Fe(OH)3 + NaCI
P+ O2 P205
Lead(II) hydroxide reacts with hydrogen chloride to produce water and lead(II) chloride.
Zinc sulfide reacts with aluminum phosphide to produce zinc phosphide and aluminum sulfide.
тт
3 (12pt)
T
只i y
Arial
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the reaction 3X + 2Y→ 5C + 4D How many moles of X are needed to produce 24.00 moles of D? If your answer is not a whole number, answer to the hundredths place.arrow_forwardOne way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. Any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a 200. mL sample of groundwater known to be contaminated with cadmium chloride, which would react with silver nitrate solution like this: CdCl₂(aq) + 2 AgNO3(aq) → 2 AgCl(s) + Cd(NO3)₂(aq) The chemist adds 75.0 mM silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He finds he has collected 7.0 mg of silver chloride. Calculate the concentration of cadmium chloride contaminant in the original groundwater sample. Be sure your answer has the correct number of significant digits. mg Larrow_forwardThere are two steps in the extraction of copper metal from chalcocite, a copper ore. In the first step, copper(I) sulfide and oxygen react to form copper(I) oxide and sulfur dioxide: 2Cu,S(s) 30,(8) 2Cu,0(s) + 2 SO, (g) In the second step, copper(I) oxide and carbon react to form copper and carbon monoxide: Cu,O(s) + C(s) 2Cu(s) + Co (g) Suppose the yield of the first step is 84.% and the yield of the second step is 95.%. Calculate the mass of copper(I) sulfide required to make 4.0 kg of copper. Be sure your answer has a unit symbol, if needed, and is rounded to the correct number of significant digits.arrow_forward
- Name: Balancing Chemical Equations Balance the following equations using whichever method you prefer: 1) K (s) + MgBr2 (aq) → KBr (aq) + Mg (s) 2) CHSOH (I) + CO2 (g) + H20 (g) 3) P (s) + (a) 30 P20s (s) 4) CO: (g) + H2O (1) > O2 (g) + CH12O6 (s)arrow_forwardBalance the following chemical equation (if necessary):arrow_forwardPredict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced and contains state symbols after every reactant and product. HCIO (aq) + H2O(l) → ローロ Х 5 2 00 Ararrow_forward
- Predict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. [] → Ba(C1O₂)₂(aq) + H₂O(1) 2 X Garrow_forwardPredict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. [[] → BaCl₂(aq) + H₂O(1) X Sarrow_forwardpeact Baking soda is often included in recipes to make food rise. When heated, it will produce sodium carbonate, water vapor, and carbon dioxide. Which of the five types of chemical reactions does the equation represent? Provide evidence and reasoningarrow_forward
- Standard airbags for automobiles used to implement the formation of sodium azide as a source of nitrogen gas. This has since been replaced due to the potential caustic nature of the products. The sodium azide required for automobile air bags is made by the reaction of sodium metal with dinitrogen oxide in liquid ammonia: 3N2O(g) + 4Na(s) + NH3(l)→ NaN3(s) + 3NaOH(s) + 2N2(g) a. You have 65.0 g of sodium and a 35.0 L flask containing N2O gas with a pressure of 2.12 atm at 296 K. What is the maximum possible yield (in grams) of the NaN3?arrow_forwardConsider the general chemical equation 3A+B 2C.arrow_forwardChloramine is a toxic gas that can form in swimming pools by the reaction of hypochlorous acid, used to sanitize pool water, with ammonia, a component of sweat and urine. Chloramine gas, NH₂Cl(g), is the cause of the identifiable 'pool smell'. The balanced chemical equation for the reaction of hypochlorous acid and ammonia is: HOCl(aq) + NH₃(aq) → NH₂Cl(g) + H₂O(l) A swimming pool holding 9.50 × 10⁴ gallons of water. 1.00 gallons HOCl sanitizer is enough to sanitize 1.00 × 10⁴ gallons of pool water. The sanitizer has a concentration of 12.00 mg/L of Cl atoms. Determine the moles of chloramine are produced if ammonia is in excess. (1 gal = 3.785 L)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY