
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
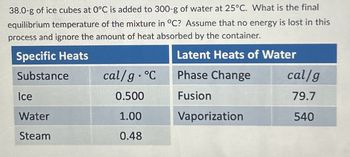
Transcribed Image Text:38.0-g of ice cubes at 0°C is added to 300-g of water at 25°C. What is the final
equilibrium temperature of the mixture in °C? Assume that no energy is lost in this
process and ignore the amount of heat absorbed by the container.
Specific Heats
Latent Heats of Water
Substance
cal/g.°C
Phase Change
cal/g
Ice
0.500
Fusion
79.7
Water
1.00
Vaporization
540
Steam
0.48
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Calculate the latent heat of vapourisation for water at 54°C using the data contained in the table below. Temperature /°C p(sat)/kPa Saturated Liquid Volume/cm^3g^-1 Saturated Vapour Volume/cm^3g^-1 53 14.29 1.014 10490 54 15.00 1.014 10020 55 15.74 1.015 9577.9arrow_forwardA 0.065-kg ice cube at -30.0°C is placed in 0.375 kg of 35.0°C water in a very well-insulated container. The latent heat of fusion for water is Lf = 79.8 kcal/kg Chart attached. What is the final temperature of the water, in degrees Celsius?arrow_forwardPlease asaparrow_forward
- A 117-g cube of ice at 0°C is dropped into 1.0 kg of water that was originally at 79°C. What is the final temperature of the water after the ice has melted?arrow_forwardThe boiling point of a liquid is 28.3°C. A portion of the liquid is at equilibrium with its vapor at 22.3°C in a sealed flask. The temperature rises to to 32.6°C.What happens inside the container? Liquid is converted to vapor, the new equilbrium mixture contains less liquid and more vapor. The vapor is all converted to liquid. Vapor is converted to liquid, the new equilibrium mixture contains more liquid and less vapor. The liquid is all converted to vapor.arrow_forwardWater at 16.0 °C is sprayed onto 0.230 kg of molten gold at 1063 °C (its melting point). The water boils away, forming steam at 100.0 °C and leaving solid gold at 1063 °C. What is the minimum mass of water that must be used? mw =arrow_forward
- Equal masses of substance A at 98.1°C and substance B at 26.2°C are placed in a well-insulated container of negligible mass and allowed to come to equilibrium. If the equilibrium temperature is 77.4°C, which substance has the larger specific heat? a) substance A b) The answer depends on the exact initial temperatures. c) More information is required. d) The specific heats are identical.arrow_forwardA jet of steam at 100°C is applied to a 2.00 kg block of ice at 0°C. How many grams of steam would be required to completely melt the ice assuming a complete transfer of thermal energy? nA d bloow olle boutaig erd 15000 lo yolla an ai e W lobin bna 1900 lo yolla ns e losbinom 4arrow_forwardA(n) 80-g ice cube at 0°C is placed in 990 g of water at 22°C. What is the final temperature of the mixture? °Carrow_forward
- One mole of oxygen gas is at a pressure of 5.45 atm and a temperature of 27.5°C. (a) If the gas is heated at constant volume until the pressure triples, what is the final temperature? °C (b) If the gas is heated so that both the pressure and volume are doubled, what is the final temperature? °C Submit Answerarrow_forwardA 1.50-kg iron horseshoe initially at 650°C is dropped into a bucket containing 13.0 kg of water at 22.0°C. What is the final temperature of the water-horseshoe system? Ignore the heat capacity of the container and assume a negligible amount of water boils away. °Carrow_forward275 g of cold water at 5.00 °C is mixed with 125 g of hot water at 80.0 °C. Assuming no energy is lost, what is the final temperature of the mixture? 21.6°C O 37.5 °C Ⓒ 28.4 °C 56.6 °C QUESTION 13 75 g of metal at 100 °C is mixed with 250 g of water at 20 °C and reaches an equilibrium temperature of 25 °C. What was the specific heat of the metal? 0.049 cal/g °C 9.8x10-3 cal/g °C 1.5 cal/g °C 0.22 cal/g °carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON