
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
(33) Please help me answer this problem. Thank you very much
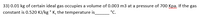
Transcribed Image Text:33) 0.01 kg of certain ideal gas occupies a volume of 0.003 m3 at a pressure of 700 Kpa, If the gas
constant is 0.520 KJ/kg ° K, the temperature is
°C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 6. (20 pts) The specific heat of a certain ideal gas is given as a function of 4.13 x 10* KJ 345.1 temperature: Cp = [1.54 - when temperature increases T kg K from 325°K to 1,100°K. Find the change of enthalpy. (Ans: 862.21arrow_forwardGiven information is attached. A. Estimate the temperature of the saturated steam at 15.0 bar. (In degrees Celsius) B. Estimate the temperature of the superheated steam at 20.0 bar.arrow_forward6. A 1.5m tank containing a one-third kilogram of an unknown ideal gas is pressurized at 200 kPaa. What is the unknown gas in the tank if the inside temperature is 160°C?arrow_forward
- 5. At temperature t= 15°C and pressure 0.98 bar, the specific volume of 0.45 m/kg. Subsequently pressure drops to P2 =0.6 bar while the temperature remains constant. Make calculation for the density of gas under the change gas %3D condition.arrow_forward2) Water in a pressure cooker is observed to boil at 175°C. What is the absolute pressure in the pressure cooker, in kPa?arrow_forward5. A copper calorimeter can with mass 0.446 kg contains 0.0950 kg of ice. The system is initially at 0.0 C. (a) If 0.0350 kg of steam at 100.09C and 1.00 atm pressure is added to the can, what is the final temperature of the calorimeter can and its contents? (b) At the final temperature, how many kilograms are there of ice, how many of liquid water, and how many of steam? 6. The operating temperature of a tungsten filament in an incandescent light bulb is 2450 K, and its emissivity is 0.350. Find the surface area of the filament of a 150-W bulb if all the electrical energy consumed by the bulb is radiated by the filament as electromagnetic waves. (Only a fraction of the radiation appears as visible light.)arrow_forward
- A certain ideal gas has the following properties: Cpo = A + (B/T) where A and B are constants and T is temperature in °K and Cpo has units kJ / K /kg. The gas constant is R = 0.25 kJ/K/kg and the constants A = 1.25, B = 173.8029748. For T₁ = 300 °K and T₂ = 400 °K, determine the change in specific internal energy, (u₂-u₁ ) in kJ/kg. You may NOT assume constant specific heats.arrow_forwardQ5) A mass of 0.1 kg of helium fills a 0.2 m' rigid vessel at 350 kPa. The vessel is heated until the pressure is 700 kpa. Calculate the temperature change of helium (in °C and K) as a result of this heating. [337°C, 337 K]arrow_forwardAnswer the highlighted partarrow_forward
- An insulated, rigid tank is divided into two equal parts by a membrane. (Figure 1) At the beginning, one part contains 9 kg of nitrogen at 500 kPa and 50 °C, while the other part is completely evacuated. The membrane is punctured and the Part A gas expands into the entire tank. Use the PG model. Determine the final temperature (T3). Express your answer to the nearest integer. HA Value Units Submit Request Answer Part B Determine the final pressure (p2 ). Express your answer to the nearest integer. ΑΣφ vec kPa Submit Request Answerarrow_forward1-arrow_forward3. Estimate of steam at 100 lbf/in2 and 400°F consider a perfect-gas approximation. (3 DECIMAL PLACES IN FINAL ANSWER PLS)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY