
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
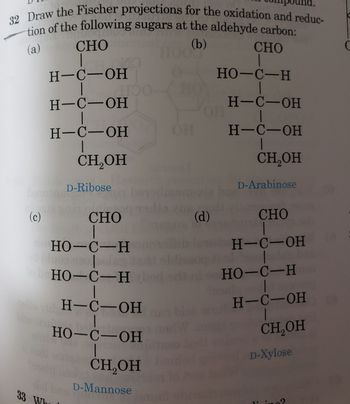
Transcribed Image Text:32 Draw the Fischer projections for the oxidation and reduc-
tion of the following sugars at the aldehyde carbon:
mos
(a)
(b)
CHO
|
HO-C-H
be
CHO
H-C-OH
H-C-OH
H-C-OH
CH₂OH
D-Ribose
dol
33 Wh
(c)
98 HO-C-H
HOO
CHO
loboHO-C-H
-
H-C-OH
1
HO-C-OH
CH₂OH
D-Mannose
H-C-OH
OH H-C-OH
|
CH₂OH
andi
(d)
D-Arabinose
CHO
H-C-OH
HO-C-H
1
H-C-OH
CH₂OH
D-Xylose
ing?
C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 10. What is the relationship between the two molecule shown below? Jon An -OH -H H OH OA) Enantiomers OB) Meso Compounds C) Constitutional Isomers D) Diastereomers E) Identical Structuresarrow_forwardHO-H HO -H Which enantiomer is the following structure?' HO -OH H -OH OH ○ alpha OD it is not an enantiomer OL ○ betaarrow_forwardt of pt pt pt fpt H- H- H- -OH -OH -OH CH₂OPO,² Ribose 5-phosphate Arrow-pushing Instructions XW H-N-Enzyme H-C CHO "tom H- -OH Conversion of ribose 5-phosphate to glyceraldehyde 3-phosphate (see above) is a step in the pentose phosphate degradation pathway. Write a detailed mechanism for the reaction above, then draw curved arrows to depict electron reorganization for step 5, the first step in hydrolysis of the iminium group below. Draw arrows to give enolates in the oxide resonance form. H-C-0-H H CH₂OPO,² Glyceraldehyde 3-phosophate + :0-H H CHO CH₂OPO, Previousarrow_forward
- which if any, of the following structures represent mesl compouds? (Blue 5 N , green 5 Cl.)arrow_forwardWhich shows the chair conformation of B-D-glucopyranose (Fisher projection shown below)? CHO H-OH HO-H H- -ОН H- -ОН ČH2OH OH OH HO ÓH OH OH OH Но- OH Он HỌ HO- HO он Но OH OHarrow_forwardH-C -OH CH₂OPO₂²- 3-phosphoglycerate ATP ADP Arrow-pushing Instructions AC XT H-C-OH :O: CH₂OPO3²- =C² H-C-OH -O OPO3² Enz-SH 3 CH₂OPO3²- 1,3-bisphosphoglycerate ADP PO S-Enz H-C-OH CH₂OPO3²- One step in the gluconeogenesis pathway for the biosynthesis of glucose is the partial reduction of 3-phosphoglycerate to give glyceraldehyde 3-phosphate. The process occurs by phosphorylation with ATP to give 1,3-bisphosphoglycerate, reaction with a thiol group on the enzyme to give an enzyme-bound thioester, and reduction with NADH. Draw curved arrows to show the movement of electrons in this step of the reaction mechanism. [Enzyme-bound thioester] NADH/H* NAD*, Enz-SH H H-C-OH CH₂OPO3²- Glyceraldehyde 3-phosphate :Ö: H-C-OH CH₂OPO3²- ADP 29arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY