
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
According to the Woodward-Hoffmann rules, is this cycloreversion reaction possible under these conditions?
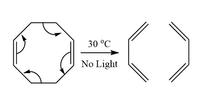
Transcribed Image Text:30 °C
No Light
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Bromination can occur in a 1,4 fashion across conjugated double bonds, as shown here for cyclohexa-1,3-diene. One mechanism that has been proposed involves a five-membered ring, bromonium ion intermediate, as shown below. (a) According to this mechanism, what should the stereochemistry be for the products-namely, all cis, all trans, or a mixture of both? (b) Observations from experiment show that both cis and trans products are formed. Does this support or discredit the proposed mechanism shown here? Br Br2 Cyclohexa-1,3-diene Br :Br: Br: Br:arrow_forwardConsider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Involves a carbocation intermediatearrow_forwardYou have learned how secondary alkyl halides are usually good substrates for SN1 and SN2 reactions. Chlorocyclopropane, however, will not react under either of the following conditions: (a) sodium methoxide in methanol or (b) methanol. Explain non-verbally why the two conversions fail.arrow_forward
- Give explanation of the correct option and explanation of the incorrect options.arrow_forwardThe diene lactone shown in part (a) has one electron-donating group (¬OR) and one electron-withdrawing group(C“O). This diene lactone is sufficiently electron-rich to serve as the diene in a Diels–Alder reaction.(a) What product would you expect to form when this diene reacts with methyl acetylenecarboxylate, a strong dienophile?arrow_forward3c)Referring to the intermediates you drew in problem below explain in detail why no meta product is obtained in the Friedel-Crafts alkylation of chlorobenzene. Draw all pertinent resonance structures to support your argument.arrow_forward
- Using one or more of the following compounds as starting materials or products illustrate, with an example, each of the following type of pericyclic reactions. Your answer should include curved arrow mechanisms. (i) a disrotatory thermal electrocyclic reaction (ii) a conrotatory photochemical electrocyclic reaction (iii) a [4+2] cycloaddition (iv) a [3,3]-sigmatropic rearrangementarrow_forward(a) Write the products of following reactions. Give mechanism with proper stereochemistry? (2 x 2.5 = 5) (ii) Me Δ Δ CNarrow_forward(a) In an acid-catalyzed hydration, one of the following 10 carbon alkynes is expected to produce a single hydration product? Select the correct alkyne and draw the structure of the hydration product that is formed from this alkyne. (I) 2-decyne; (II) 3-decyne; (III) 4-decyne; (IV) 5-decyne; (V) none of these will give a single hydration product. (b) The reaction shown below gives Compound X as the major product. The mass spectrum of X is shown. Br2, H20 Compound X 100 - MS-IW-5644 80 60 40 - 20 - 20 40 60 80 100 120 140 160 180 200 220 m/z Considering the reactions of alkynes and the MS data, de duce which of following structures corresponds to X: Br Br HO, IV V I II II Support your answer with a reaction mechanism for fomation of X and identification of relevant peaks in the mass spectrum. 12 Relative Intensityarrow_forward
- Following is an equation for hydroperoxidation of cumene. light + O2 for HOO- Cumene Cumene hydroperoxide Propose a radical chain mechanism for this reaction. Assume that initiation is by an unspecified radical, R.arrow_forwardThe reaction between singlet oxygen and limonene (below) is often used to characterize the behaviour of singlet oxygen as an electrophile rather than an initiator for a free radical mechanism. HOO Limonene Scheme 1 (a) Since singlet oxygen is a high energy state, the formation of a diradical seems plausible: 0=0* Propose a sequence of steps for this diradical reacting with limonene and explain why the major product, which is obtained (Scheme 1), does not support this reaction mechanismarrow_forward(c) Compound 2F shown below is a tetra-substituted cyclooctatetraene. After heating 2F at 160 degree for 6 hours, researchers observed the formation of an isomer of 2F. Draw the isomer structure and provide a plausible mechanism to explain the formation of the isomers. 2Farrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY