
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I only need help with questions three, please solve but always explain. I am not sure what the question is asking, and no other techniques come to mind when thinking about how to dry crystals more.
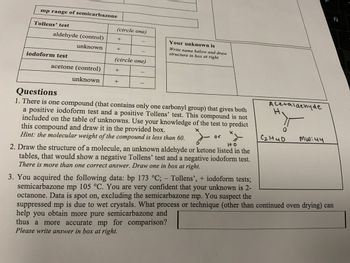
Transcribed Image Text:mp range of semicarbazone
Tollens' test
aldehyde (control)
unknown
iodoform test
acetone (control)
unknown
(circle one)
+
+
+
-
(circle one)
+
-
-
1
Your unknown is
Write name below and draw
structure in box at right
Questions
1. There is one compound (that contains only one carbonyl group) that gives both
a positive iodoform test and a positive Tollens' test. This compound is not
included on the table of unknowns. Use your knowledge of the test to predict
this compound and draw it in the provided box.
Hint: the molecular weight of the compound is less than 60.
HO
2. Draw the structure of a molecule, an unknown aldehyde or ketone listed in the
tables, that would show a negative Tollens' test and a negative iodoform test.
There is more than one correct answer. Draw one in box at right.
Acetaldehyde
H
여
Санио
MW:44
2
Ñ
3. You acquired the following data: bp 173 °C; - Tollens', + iodoform tests;
semicarbazone mp 105 °C. You are very confident that your unknown is 2-
octanone. Data is spot on, excluding the semicarbazone mp. You suspect the
suppressed mp is due to wet crystals. What process or technique (other than continued oven drying) can
help you obtain more pure semicarbazone and
thus a more accurate mp for comparison?
Please write answer in box at right.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In 500. g of water at 50 degrees Celsius, approximately what mass of substance C will dissolve? Round your answer to a whole number.arrow_forwardA student mistakenly uses 0.31g Mg. Will the temperature change of the solution be affected/ If so, will it be larger or smaller that it would be have been using 0.15g mg? Explain. The amount of heat released is (independent of the quality, a molar quality, depends on the temperature)arrow_forwardWhich of the processes can help solids dissolve faster? decrease the amount of solvent decrease the temperature of solution increase the temperature of solution increase the amount of solutearrow_forward
- powders and granules dosage forms have faster dissolution rate than capsules and tablets ,why?arrow_forwardWhat is hard water? What are the main problems combining soap and hard water?arrow_forwardA mixture of copper and tin, commonly called bronze is a homogeneous mixture or a ____________ solution.arrow_forward
- Why is it best to cool a solution slowly as a solid crystallizes? impurities are more likely to crystallize as well when done quickly the solid obtained will have a higher purity when done quickly no solid will form when done quickly the solvent could start to boil again when done quicklyarrow_forwardWhy does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?arrow_forwardB. SOLID TO GAS Place a few crystals of iodine in a dry test tube. Heat the tube and note the changes. Cool the tube. Observations: What happens when the tube is heated? What is this substance? Does any iodine melt? What is formed on cooling the tube? What is this process called? Define it. Name another substance that could undergo such a process.arrow_forward
- Explain why air is classified as a solution and oxygen is classified as a pure substance.arrow_forwardDescribe why adding a solvent would either lower the vaper pressure of raise the boiling point.arrow_forwardDetermine if crystalline sodium chloride and water are incompatible. If they are incompatible, state the hazardous conditions which would result from mixing the wastes.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY