
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
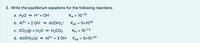
Transcribed Image Text:3. Write the equilibrium equations for the following reactions.
а. Н2О Н+ + ОН-
Kw = 10-14
%3D
b. Al3+ + 2 Онө Al(ОН)2*
Ка2 3D 5x1018
с. СО2(g) + Н20 НасОз
Кн 3 10-1.5
%D
d. Al(OH)3(s) АB+ + 3 Он-
Ksp = 3x10-34
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 7 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. The shape of boron trihydride, BH3, is 2. In an exothermic reaction, the potential energy of the products is than the potential energy of the reactants. 3. A system at equilibrium always has forward and reverse reaction rates which are 4. A solution at 25°C with a pOH of 3.45 has a pH of 5. When nitric acid is titrated to an end point by lithium hydroxide, two products are and O 6. The standard reduction potential table lists the Evalues of half-cell reactions measured in combination with the standard half-cell.arrow_forwardWhat is the correct equilibrium constant expression for the balanced reaction shown below? PC13 (9) + PBr3 (9) PCl2Br (g) + PCIBr2(g) [PCl3] [PBr3] [PCl₂ Br][PCIBr₂] [PCIBr₂][PCl₂Br] [PCl3] [PBr3] [PCIBr₂][PCl3][PBr3] [PCl₂Br] [PCIBr₂]²[PCl₂Br]² [PCl3] [PBr3] [PCl3][PBr3] [PCIBr₂]²[PCl₂ Br]² O a. a O b.b OC.C O d.d O e.e a b C d earrow_forward20. What will happen to the equilibrium when more SO2 (g) is added to the following system? 2502(g) + O2(g) = 250(g) A. It will shift to the right. B. It will shift to the left C. no shift of equilibrium D. cannot be determined 21. For the reaction 2A+B2C the appropriate form for the equilibrium constant expression is: A. [A][B12/ [C] B. [AB]/[C12 C. [C]2/[A][B] D. [ABRIC] 22. Calculate K for the following reaction given the following equilibrium concentrations of H2, CO, and H₂O. PCl3(g) + Cl₂(g) K=? Equilibrium Concentrations (M): PCIs(g) .200 .040 .080 A. 0.16 B.0.016 C.1.6 D.16 23. Which of the following is not omitted when writing the equilibrium constant expression? D. all of the choices A. gas C. solid B. liquid 24. When can we say that the reaction is already at equilibrium? A. When Q is not equal to K. C. When Q is greater than K. D. When Q is lesser than K. B. When Q is equal to K. 25. Where will the equilibrium shift when we increase the pressure of the system? B. towards…arrow_forward
- The equilibrium constant is given for one of the reactions below. Determine the value of the missing equilibrium constant. N2O4(g) = 2NO2 (g) Kc = 1.46 5N2O4(g) = 10NO2 (g) K. = ? %3D 0.322 O 5.40 O 1.46 O 1.13 O 6.63arrow_forwardConsider the exothermic reaction: A (g) + B (s) → 2X (g) @ 25 °C a. Propose a change in VOLUME so that the equilibrium position shifts towards the REACTANTS. b. Propose a change in TEMPERATURE so that the equilibrium position shifts towards the PRODUCTS. c. Propose a change in [A] so that the equilibrium position shifts towards the PRODUCTS.arrow_forwardConsider the following: N2(g)+ 3H2(g) = 2NH3(g) Ke= 6.3 × 10° What is the new K, for this reaction but I rewrite it to show the production of 1 mole of H2 and 0.333 moles of N2 from 0.666 moles of NH3? A. 2.10 x 108 B. 1.17 x 103 C. 1.36 × 103 D. 3.99 x 10-27arrow_forward
- Use Le Châtelier’s Principle to explain the effect each of the following changes will have upon the system—will the equilibrium shift toward the product or reactant side? Why? N2(g) + 3 H2(g) ↔ 2 NH3(g) + heat a. If more hydrogen is added to the system the equilibrium will shift to the..... (pick one and explain)i. Right ii. Left iii. Remains unchanged b. If ammonia is removed from the system the equilibrium will shift to the..... (pick one and explain)i. Right ii. Left iii. Remains unchanged c. If nitrogen is removed from the system the equilibrium will shift to the..... (pick one and explain)arrow_forwardGiven the following reaction what is the expression for Kc? 2SO2(g)+O2(g)->2SO3(g) a.What is the expression for the equilibrium constant? b.If the equilibrium constant for this reaction is 3.70 X 10-7, are the products or the reactants favored? c.What is the equilibrium constant for the reverse reaction? d.What is the equilibrium constant for the reaction below? 6 SO3(g)-> 6 SO2(g)+3O2(g)arrow_forwardf the value of Q is greater than K for an equilibrium reaction then a. a catalyst is necessary to achieve equilibrium. b. the reaction will proceed to the right until equilibrium is established. c. the reaction will proceed to the left until equilibrium is extablished. d. the reaction will go left or right depending on the reaction stoichiometry. e. Both (a) and (d) are correct.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY