
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
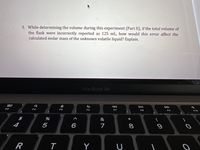
Transcribed Image Text:3. While determining the volume during this experiment (Part II), if the total volume of
the flask were incorrectly reported as 125 mL, how would this error affect the
calculated molar mass of the unknown volatile liquid? Explain.
MacBook Air
80
DII
F3
F4
F5
F6
F7
F8
F9
F10
&
*
4
5
6
7
8
R
Y
* 00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calcium phosphate (Ca3(PO4)2) is a chemical that can be added during water treatment and the chemical undergoes the following reaction when added to water. Ca3(PO4)23 Ca²+ + 2 PO³- If 37 grams of 94% pure Ca3(PO4)2 is added to 55 liters (L) of water, find the calcium (Ca²+) concentration in units of milli-moles per liter (mM). Report your answer to the nearest hundredths (0.01) place.arrow_forwardBy pipet, 9.00 mL of a 0.823 M stock solution of potassium permanganate (KMNO4) was transferred to a 50.00-mL volumetric flask and diluted to the calibration mark. Determine the molarity of the resulting solution. HA Value Unitsarrow_forwardA solution is prepared by pipetting 25.00 mL of 3.0 x 10-4 M CaCl2 into a 750.00 mL volumetric flask and then adding water to the calibration mark. What is the concentration?arrow_forward
- A student finds that they have 0.2545 g of NH4Cl, 1.1502 g of NaCl, and 1.0181 g of SiO2. Calculate the percent gained or lost during this experiment if the initial mass of the mixture was 2.5537 g.arrow_forwardFor experiment of PREPARATION OF ACETYLSALICYLIC ACID (ASPIRIN): The experiment required 3 drops of concentrated sulfuric acid. Why was this acid added? Would the reaction take place if this reagent were omitted? A student thought he carefully followed the directions in the experimental procedure, and he expected 2.63 g of product. However, his isolated product weighed 3.05 g. What would account for the excess weight? Bottles containing old aspirin tablets often smell of vinegar. The presence of what chemical causes this smell? How does this chemical form?arrow_forward1. Develop a detailed separation scheme for the separation and determination of the percent composition of your sample which will be a mixture of NaCl, NH.CL, and sand. You are expected to use the properties of the components listed below and some of the techniques listed in the table in the pre-lab queries (and used in Separations I). Keep in mind that any chemistry student should be able to pick up your scheme, understand it, and use it to complete the separation and calculation of % composition. Place the scheme on a separate sheet of paper. Component Solubility (@25°C) Melting Point Hardness sodium chloride, NaCl 35 g/100 mL water 801°C soft ammonium chloride, NH&Cl 37 g/100 mL water sublimes 350°C soft sand, SIQ2 insoluble 1600°C hard Example of compounds in a container. Naci NH.CI sio2 Exploring the Chemical World, PGCC, 2003 4 RESULTS Show all calculations with units in this space.arrow_forward
- When answering this problem, report the answer with the appropriate number of significant figures. When entering units, use proper abbreviated units with proper capitalization. A titration is performed to determine the amount of sulfuric acid, H2SO4, in a 6.5 mL sample taken from car battery. About 50 mL of water is added to the sample, and then it is titrated with 43.37 mL of a standard 0.5824 molar NaOH solution. You balanced this reaction in a previous problem. How many moles of sulfuric acid are present in the original sample? Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: ______x10 ____power_____unitsarrow_forwardHere is a graph of the molarity of acetonitrile CH3CN in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table belowarrow_forwardBased on this. What was the percentage by mass of water in the sample?arrow_forward
- Tell whether each of the following errors will cause the experimental mass percent of the sodium bicarbonate to be higher than, lower than, or the same as the true value. Explain your answer. The crucible was not heated long enough prior to adding mixture. Some of the carbonate spattered out from the crucible during heating The sample was not heated to constant mass. The mass of mixture used is greater than the recommended mass.arrow_forwardHow much 20.0% NaOH should I put in a 30.0 ml tube if my dilution ratio is 1/10. What is the concentration of the tube. ml of 20.0% Just put the number of mL Do not enter anything but a number. Use correct sig figs. Remember ratios and conversion factors do not enter the significant figure calculation. What is the concentration in percent. Again, correct significant figures. NUMBERS only not the % signarrow_forwardWhen answering this problem, report the answer with the appropriate number of significant figures. When entering units, use proper abbreviated units with proper capitalization. A titration is performed to determine the amount of sulfuric acid, H2SO4, in a 6.5 mL sample taken from car battery. About 50 mL of water is added to the sample, and then it is titrated with 43.37 mL of a standard 0.5824 molar NaOH solution. You balanced this reaction in a previous problem.How what is the molar concentration of sulfuric acid in the original sample? Present the answer in the following form. Answer: _____ x10 ___power ___unitsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY