Question
Identify the daughter nuclide, the type of decay (for natural transmutations) and the nuclear reaction
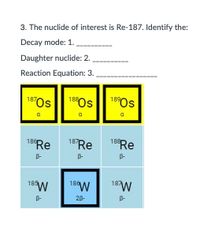
Transcribed Image Text:3. The nuclide of interest is Re-187. Identify the:
Decay mode: 1.
Daughter nuclide: 2.
Reaction Equation: 3.
187Os
189Os
188
a
a
186
187
188
B-
B-
B-
185
18W
18W
B-
28-
B-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps
