
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:3. Pick the frequency of you
the wavelength. Using th
include the units in youra
Show the steps on how you solve
Expert Solution
arrow_forward
Step 1
Radio stations are a great source of information. One can get news, entertainment etc things on radio stations. These radio stations rely some specific frequency which is received by a receiver.
arrow_forward
Step 2
Now lets us pick frequency of a radio station to be 93.5 Hz. The wave released by radio stations are radio waves. They are a type of electromagnetic waves. So they contain some sort of frequency, wavelength and energy. In electromagnetic waves, direction of propagation of wave, electric field, magnetic field are perpendicular to each other. Distance between two consecutive crest and trough is called wavelength of that wave. Its relation with frequency is as follows-
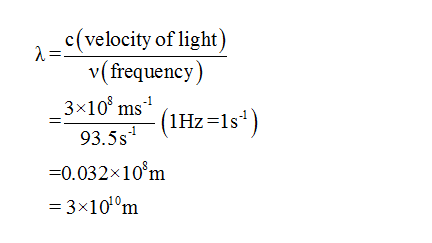
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- We are able to determine both the energy of an electron and it's location in the atom using sophisticated simultaneously instrumentation. True Falsearrow_forwardWhat hydrogen atomic orbital has: A. One radial node and one angular node? B. no radial nodes and 2 angular nodes?arrow_forwardPart B Why is the wave nature of matter not important for a baseball? Match the items in the left column to the appropriate blanks in the sentence on the right. Reset Help small The wave nature of matter is not important for a baseball because the value of the wavelength is large compared to the size of the ball and it on the trajectory of the ball. has no effect has a great effectarrow_forward
- What is the number of angular nodes in each case? a.) 1s (angular nodes) b.) 3s (angular nodes) c.) 3d (angular nodes)arrow_forwardHow many radial nodes and how many angular nodes does the orbital shown in the figure below have?arrow_forwardWhat is the label for the orbital shown here that indicates the type of orbital and its orientation in space? z Express your answer using appropriate letters (e.g., Pz). Do not include the energy level n. • View Available Hint(s) ? Submit Previous Answersarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY