
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Answers are attached. Please show all steps and make an ICE table. Thank you!
![3 [H₂0] = [N₂0] = 0.537M](https://content.bartleby.com/qna-images/question/beaa6890-e5db-44c8-b5cd-a4b86da11d95/f7bcfbab-c21e-4ee3-9031-9fd56b690cc6/dqeott8_thumbnail.png)
Transcribed Image Text:3 [H₂0] = [N₂0] = 0.537M
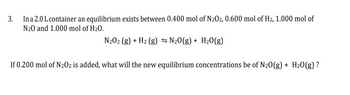
Transcribed Image Text:3.
In a 2.0 L container an equilibrium exists between 0.400 mol of N202, 0.600 mol of H₂, 1.000 mol of
N₂0 and 1.000 mol of H₂O.
N₂O2 (g) + H₂ (g) = N₂O(g) + H₂O(g)
If 0.200 mol of N₂02 is added, what will the new equilibrium concentrations be of N2O(g) + H₂O(g)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Screen Reader Description: The Ordinary Phases of Water. Image: Graph of Temp (Kelvins from 0 to 700) on X axis, P (atm) on Y axis (from 10 to the power of 0 to 10 to the power of 8, pressure, (P a)). There is a curve starting at 200 on the X axis and 10 to the power of 0 on the Y axis. The line goes upward to the right to finish at 610 on the X axis and 10 to the power of 7 on the Y axis. There is a straight line with a slightly negative slope starting at 260 on the X axis and 10 to the power of 8 on the Y axis that goes downward until it hits the curve and stops. To the left of the straight line is the word "Solid". Between the straight line on the right and the curve is the word "Liquid". To the right side of the curve is the word "Gas". End of image. The critical temperature is: 0 K 100 K 200 K 300 K 400 K 500 K 600 K 700 K 100 Pa 101 Pa…arrow_forwardI need help with question 2 please!arrow_forwardHi, what is an example of water resisting temperature change? and why?arrow_forward
- A sample of air is determined to have an NO2 concentration of 0.067 ppm. what is the concentration in micrograms/cubic meters? Assume air pressure is 1.0 atm and the temp is 290 Karrow_forwardA mixture of copper and tin, commonly called bronze is a homogeneous mixture or a ____________ solution.arrow_forwardDescribe the process by which a medical cold pack works. Make sure to include a description of the energy within the bonds of the chemicals.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY