
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
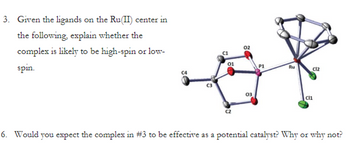
Transcribed Image Text:3. Given the ligands on the Ru(II) center in
the following, explain whether the
complex is likely to be high-spin or low-
spin.
C4
û
U
a
C2
02
03
P1
Ru
C12
Cl1
6. Would you expect the complex in #3 to be effective as a potential catalyst? Why or why not?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The CFSE for a [Re(Ox)2Br2]3 : complex is a)-0.6Aoct b)2.0 Aoct c)1.8 Aoct d)1.6 Aoct e)1.2 Aoctarrow_forward1a) Using the ionic model, determine the valence electron count of the transition metal in complex A shown below; convince yourself that complex A is already a catalyst. (i-Pr)2P- -Ir -P(i-Pr)2 Aarrow_forwardHelp me pleasearrow_forward
- 5. Determine the denticity of each ligand (i.e., is it monodentate, bidentate, etc.). H₂N HO. OH H ∙N. OH OH NH₂arrow_forwardTp 2Fe is a low-spin Fe(II) complex with Fe-N bonds of 1.97 Å. Tp Ph 2Fe is a similar complex that is high-spin Fe(II) and has Fe-N bonds of 2.25 Å. Explain why the high-spin complex would have longer (i.e., weaker) Fe-N bonds than the low-spin complex.arrow_forwardWhich of the following statements about 10Dq is false? A. In the [CoX(NH3)5]n+ complex, the color changes from dark violet to yellow when X is replaced by I-, Br-, Cl-, H2O, and NH3, respectively.B. [VCl6]2- has a greater 10Dq than [VCl4] complex.C. [MnF6]2- has a higher 10Dq value than [TcF6]2-.D. [V(H2O)6]3+ has a higher 10Dq value than [V(H2O)6]2+.arrow_forward
- Identify the first-row transition metal for the following 18-electron species: a. [M(CO)3(PPh3)]¯¯ c. (n*-CgHg)M(CO), b. HM(CO)5 d. [(n-C5H5)M(CO)3]2 (assume single M-M bond)arrow_forward1. The Cp2Rh2[u-(CF3C=CCF3)](CO)(CNR) complex has a Rh-Rh bond distance of 2.67 Å, strongly indicating a covalent bond between the rhodium atoms. How would you electron count this complex to accommodate a Rh-Rh covalent bond? Clearly show the electron counting.arrow_forwardUsing the ligands ethylenediamine (H2NCH2 CH2NH2) and bromide anions, design a cationic chiral octahedral complex of cobalt(III) with a +1 charge. Draw the coordination complex with its enantiomer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY