
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
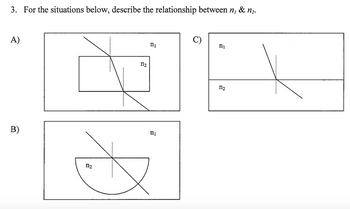
Transcribed Image Text:3. For the situations below, describe the relationship between n, & n₂.
A)
B)
112
C)
ոլ
ոլ
ni
D
ոշ
n₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 3) An ideal gas with a volume of .867 m3 has a temperature of 119°C. If there are 8.72 x 1024 particles, what is the pressure? Hint: make sure to convert particles to moles!arrow_forward2. A non-ideal gas, exhibiting strongly non-linear relationship between pressure and temperature by 1.5 polynomial degrees is expanded in an isolated chamber. The volume gas _________ A. DecreasedB. Reimained the sameC. Increasedarrow_forward6 of 13 I Review I Constants For related problem-solving tips and strategies, you may want to view a Video Tutor Solution of Volume of a gas at STP. Part A If a certain amount of ideal gas occupies a volume V at STP on earth, what would be its volume (in terms of V) on Venus, where the temperature is 911.0°C and the pressure is 91.8 atm? Express your answer as a multiple of volume V at STP. DA ΑΣφ ? Vvenus Submit Request Answerarrow_forward
- E) the Maxwell speed distribution. A certain quantity of hydrogen occupies a volume of 1000 cm² at 0 °C and ordinary atmospheric pressure. 24. If the pressure is tripled but the temperature is held constant, what will the volume of the hydrogen be? A) 3.33 m B) 33.3 m' C) 33.3 x 10° m D) 3.33 x 104 m' E) The correct answer is not givenarrow_forward1arrow_forwardM Inbox X M Insta X Insta x C Ingric X ← → CO UUtomik Games Regis x learn.maricopa.edu/courses/1241873/assignments/13104919?module_item_id=26832639 LastPass password... a Amazon.com - Onli... Express VPN 2 Homework 111.pdf 71°F Partly cloudy 75E05F70-341B-4....jpeg Amazon.com - Onli... Find x Homework Degr X Asso x x ■ Asso X Imported from Chr... X X₁ + Vit + zat² V=Vi + at Can be combined to form the equation: V₁²-V₁²= 2a (X-Xi) How xb Hom x a Page < Remember to use unit conversion and dimensional analysis to check your answers. That is, verify the units of your answer are the expected units. For the acceleration due to gravity use 9.81 (m/s)/s downward. 1) In some situations we don't have information about time when trying to solve a problem. Show that the equations: 1 of 5 Hom X ↓ Download + ℗ Info ZOOM + X Close Show all 1:14 PM 11/2/2022 X : Xarrow_forward
- 1.)The temperature rises, stays the same for a time, and then falls 2.)The temperature rises, stays the same for a time and then rises again.arrow_forwardA surveyor has a stainless steel measuring tape that is calibrated to be 100.000 m long (i.e., accurate to ±1 mm) at 20°C. She needs to add the correction factor to get the true distance. Submit Correct Part B How large, in mm, is the correction factor? Express your answer in millimeters to two significant figures. ► View Available Hint(s) Previous Answers AL = 11 Submit VΠ ΑΣΦ Provide Feedback Previous Answers ? X Incorrect; Try Again; 14 attempts remaining mmarrow_forwardProblem 1: Answer the following questions using the table to the right. Medium Gases at 0 °C, 1 atm Air Carbon dioxide Hydrogen Oxygen 1.000293 1.00045 1.000139 1.000271 Liquids at 20 °C Benzene Carbon disulfide Carbon tetrachloride Ethanol Glycerine Water, fresh 1.501 1.628 1.461 1.361 1.473 1.333 Solids at 20 °C Diamond Fluorite Glass, crown 2.419 1.434 1.52 Glass, flint |Ice (at 0 °C) Polystyrene Plexiglas Quartz, crystalline Quartz, fused Sodium chloride 1.66 1.309 1.49 1.51 1.544 1.458 1.544 Zircon 1.923 Part (a) Calculate the index of refraction for a medium in which the speed of light is 2.012 × 10°. Numeric : Anumeric value is expected and not an expression. Part (b) Determine the most likely identity of the substance based on the table in the problem statement. MultipleChoice : 1) Carbon tetrachloride 2) Diamond 3) Ethanol Sodium chloride 5) Plexiglass 6) Water, fresh 7) Glycerine 8) Polystyrene 9) Glass, crown 10) Glass, flintarrow_forward
- I am not sure about the answer, please tell me which one is the correct answer.arrow_forwardDirections: Solve the following problems. Use g = 9.80 m/s? and assume all numbers are accurate to 3 significant figures unless otherwise indicated. 1. A cylinder that has a 41.0-cm radius and is 50.0 cm deep is filled with air at 29.5°C and 1.00 atm shown in figure (a). A 21.0-kg piston is now lowered into the cylinder, compressing the air trapped inside as it takes equilibrium height hi as shown in figure (b). Finally, a 21.5-kg dog stands on the piston, further compressing the air, which remains at 29.5°C as shown in figure (c). What is the value Ah? Ah 50.0 cm h;arrow_forward1 please need help, keep in mind the 7 basic units and watch the sig figs. Please write in standard form need to see all the numbers in solution thank you so mucharrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON