
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:# Reaction Equilibrium Analysis
**Objective:** Determine the reaction quotient and predict the direction that each of the following reactions will proceed to reach equilibrium.
### Reactions and Conditions:
**1. Reaction 1:**
- **Equation:** \( 2 \text{NO}(g) + \text{Cl}_2(g) \rightleftharpoons 2 \text{NOCl}(g) \)
- **Conditions:** A 1.00 L flask containing 0.1500 mol NO, 0.0155 mol Cl\(_2\), and 0.500 mol NOCl.
- **Equilibrium Constant (K\(_{\text{eq}}\)):** \( 4.16 \times 10^{-5} \)
- **Prediction:** Shift right.
**2. Reaction 2:**
- **Equation:** \( \text{N}_2(g) + 3\text{H}_2(g) \rightleftharpoons 2\text{NH}_3(g) \)
- **Conditions:** A 500 L flask containing 14 g N\(_2\), 12 g H\(_2\), and 17 g NH\(_3\).
- **Equilibrium Constant (K\(_{\text{eq}}\)):** 0.060 (at 526°C)
- **Prediction:** Shift left.
**3. Reaction 3:**
- **Equation:** \( 2\text{SO}_2(g) \rightleftharpoons 2\text{SO}_3(g) + \text{O}_2(g) \)
- **Conditions:** A 200 L flask containing 230 g SO\(_3\).
- **Equilibrium Constant (K\(_{\text{eq}}\)):** 0.230
### Prediction:
**General Approach:**
1. **Calculate the Reaction Quotient (Q):** Compare Q to K\(_{\text{eq}}\) to predict the direction of the shift.
2. **Interpret the Shift:**
- If \( Q < K\), the reaction will shift right (toward products).
- If \( Q > K\), the reaction will shift left (toward reactants).
### Conclusion for Reaction 3:
- **Prediction:** The specific shift is not provided; calculate Q and compare with K\(_{\text{eq}}\) to predict the direction.
Use this analysis
Expert Solution
arrow_forward
Step 1
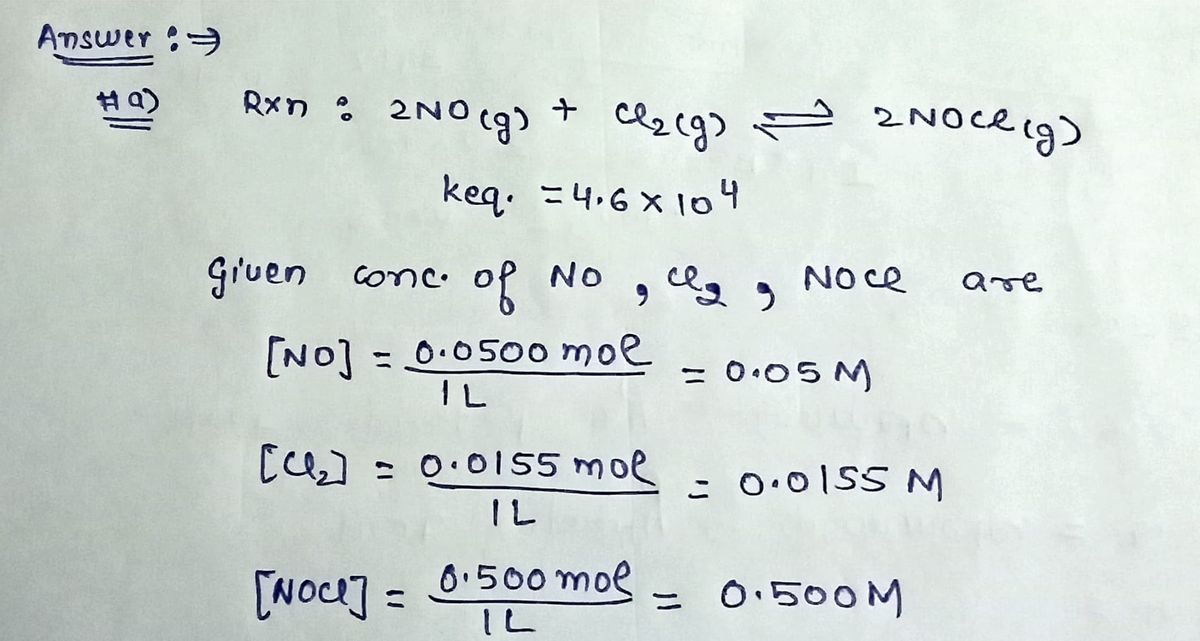
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The equilibrium constant is 0.0900 at 25°C for the reaction H₂O(g) + Cl₂O(g) → 2 HOCI(g) For which of the following sets of conditions is the system at equilibrium? For those that are not at equilibrium, in which direction will the system shift? a. A 1.0-L flask contains 4.0 mole of HOCI, 0.40 mole of Cl₂O, and 0.40 mole of H₂O. ŵ b. A 2.0-L flask contains 0.084 mole of HOCI, 0.080 mole of Cl₂O, and 0.98 mole of H₂O. Submit Answer ↑ c. A 3.0-L flask contains 0.38 mole of HOCI, 0.0010 mole of Cl₂O, and 0.47 mole of H₂O. ✰ Try Another Version 1 item attempt remainingarrow_forward13.arrow_forwardConsider the following reaction where K. = 83.3 at 500 K. PCI3(g) + Cl2(g) PCI5(g) A reaction mixture was found to contain 2.54×10-2 moles of PCI3(g), 3.91×10-2 moles of Cl2(g) and 0.115 moles of PCI5(g), in a 1.00 liter container. Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qc, equals The reaction A. must run in the forward direction to reach equilibrium. B. must run in the reverse direction to reach equilibrium. C. is at equilibrium.arrow_forward
- Consider the following reaction where Kc = 154 at 298 K. 2NO(g) + Br₂(g) —2NOBr(g) A reaction mixture was found to contain 2.48×10-2 moles of NO(g), 3.91×10-2 moles of Br₂(g) and 8.22x10-2 moles of NOBr(g), in a 1.00 liter container. Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qc, equals The reaction A. must run in the forward direction to reach equilibrium. B. must run in the reverse direction to reach equilibrium. C. is at equilibrium. Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardPhosphorus pentachloride decomposes at higher temperatures. PC15(g) → PCl3(g) + Cl₂(g) Z An equilibrium mixture at some temperature consists of 5.77 g PCL5, 208.23 g/mol 4.86 g PC|3, 137.33 g/mol 3.59 g Cl₂, 70.91 g/mol in a 1.00-L flask. If you add 1.31 g of Cl₂, how will the equilibrium be affected and what will the concentration of PCI5 be when equilibrium is reestablished? shift left shift right no shift will occur [PCI5] = 0.0276 mol/Larrow_forwardGiven K, and all but one of the equilibrium concentrations, find the concentration of the one that was not given. Example: Z2 D 2 Z An equilibrium mixture of Z2 and Z has [Z] = 0.93 M. Find [Z2], given that the value of K is 5.00. Given K and the initial concentrations of all reactants and products, find the final (equilibrium) concentrations. Example: Z2 D 2 Z initial concs: 0.400M 0.000M Given K and the concentrations of all reactants and products, determine if the reaction is at equilibrium. If it is not, determine which way the reaction will go. In the hardest version of this problem type, determine the equilibrium concentrations. Example: Is the system below at equilibrium? (K is equal to 5.00.) If not, which way will the reaction go? Find the equilibrium concentrations. Z2 D 2 Z concs: 2.00 M…arrow_forward
- Please helparrow_forwardConsider the following reaction: SO2Cl2(g)⇌SO2(g)+Cl2(g)SO2Cl2(g)⇌SO2(g)+Cl2(g) A reaction mixture is made containing an initial [SO2Cl2][SO2Cl2] of 2.3×10−2 MM . At equilibrium, [Cl2]=[Cl2]= 1.2×10−2 MM . Calculate the value of the equilibrium constant (Kc)(Kc). Express your answer to two significant figures.arrow_forwardFor the reaction at equilibrium: 2 H2O (g) +2 Cl 2 (g) + energy4 HCI (g) + O2 (g), If the temperature in the reaction vessel is decreased: O [H2O] will decrease. [H2O] will increase. [H2O] will not change.arrow_forward
- NAME: COC12(g) has a 8. At 100.0°C, the equilibrium constant for the reaction: COg) + Cl2(g) value of 4.6 x 10°, If 0.40 mol of COC12 is placed into a 10.0 L flask at 100.0°C, what will be the equilibrium concentration of all species? (A simplifying approximation that will make the solution of the resulting equation easier is to note that X is much less than 0.040 mol/L. This means that 0.040-x is approximately 0.040.)arrow_forward1. In your own words, explain the difference between the equilibrium constant, K, and the reaction quotient, Q. How do we use K and Q to determine what will happen to a specific reaction under a given set of conditions?arrow_forwardConsider the reaction. Br2(g)+Cl2(g)⇌2BrCl(g) Kp=1.11×10−4 at 150 K A reaction mixture initially contains a Br2 partial pressure of 755 torr and a Cl2 partial pressure of 735 torr at 150 K. Calculate the equilibrium partial pressure of BrCl.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY