
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Give typed explanation not a single word hand written otherwise leave it
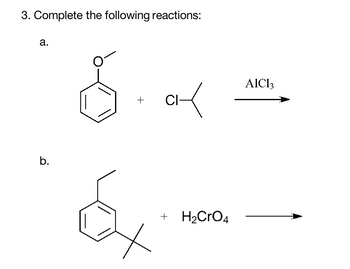
Transcribed Image Text:3. Complete the following reactions:
a.
b.
+
✓
CI-
+ H₂CRO4
AIC13
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Willa weighs a clean, dry, empty crucible and finds it to have a mass of 95.83 g. After putting a spoonful of an unknown hydrate into it, she finds that the mass has increased slightly to 99.87 g. She heats the crucible and its contents, and finds that the mass has dropped to 97.22 g. Willa is told by her teacher that the molar mass of the anhydrous salt is 75.65 g.arrow_forwardPlease don't provide handwriting solutionarrow_forwardCH,2, Zn(Cu) Et,0 CH,l,, Zn(Cu) Et,0arrow_forward
- Predict the chemical formulas of the compounds formed by the following pairs of ions.arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardhydrogen peroxide → water + oxygen List two physical / chemical properties for each compoundarrow_forward
- Here are the common abbreviation of compounds found in commercial products and thelaboratory. Find out what they mean. Abbreviation Name / Meaning Chemical Formula MSG (added in food to give an umami flavor) PVC ( polymer used in making pipes) DCM (solvent used in the laboratory) TMA (one of the components fishy odor of seafoods) HDPE (use in making plastic bottles in the lab)arrow_forwardYou wish to prepare 237 grams of 26.7% (w/w) KI. Assume that the density of water is 1.00 g/mL. You will need grams of potassium iodide and mL of water.arrow_forwardGive the formula for the compound formed when potassium oxide reacts with water. Express your answer as a chemical formula.arrow_forward
- Can you please please rewrite the question in your own words to clarify it, also write a useful hint to help you understand this problem and Provide the correct answer? Show the calculation and briefly describe the steps At the very end, write a paragraph addressing any knowledge/skill gaps, deficiencies in how you approach studying, and specifics on how you will improve Please answer fast and please try to answer allarrow_forwardNeed help from A-Darrow_forwardAssume the observable Universe is charge neutral, and that it contains n nuclei (hydrogen plus helium nuclei, ignoring other elements). Take the helium mass fraction as 1/4. How many electrons are there in the observable Universe? Enter your answer in scientific notation with one decimal place. n = 7*10^80arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY