Question
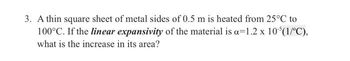
Transcribed Image Text:3. A thin square sheet of metal sides of 0.5 m is heated from 25°C to
100°C. If the linear expansivity of the material is a 1.2 x 10³(1/°C),
what is the increase in its area?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- 1. You are looking to purchase a small piece of land in Hong Kong. The price is "only" $ 62000 per square meter! The land title says the dimensions are 21 m x 30 m. By how much would the total price change if you measured the parcel with a steel tape measure on a day when the temperature was 20 °C above normal? The coefficient of linear exapansion of steel is a = 1.2x10-5/°C. The price by $ increases decreasesarrow_forward15.arrow_forward1. A quartz cube has sides equal to 18.0 cm. What will be its change in volume if its temperature is increased by 275 °F? (the coefficient of volume expansion of quartz is 1.50 × 10–0 °c•1) cm3 ssf60 160 ssfarrow_forward
- 12. At 20°C, an aluminum (αAI = 24.0 x 106 °C-¹) ring has an inner diameter of 5.0000 cm and a brass (αBr = 19.0 x 10-6 °C-¹) rod has diameter of 5.0500 cm. a) If only the ring is heated, what temperature must it reach so that it will just slip over the rod? [437°C] b) What if? If both are heated together, what temperature must they both reach so that the ring just slips over the rod? Would this latter process work? Why? [2100°C, but this will not work because aluminum melts at 660°C]arrow_forward2. An iron ring is to fit snugly on a cylindrical iron rod. At 20 C, the diameter of the rod is 6.445 cm and the inside diameter of the ring is 6.420 cm. To slip over the rod, the ring must be slightly larger than the rod diameter by about 0.008 cm. To what temperature must the ring be brought if its hole is to be large enough so it will slip over the rod?arrow_forward3. A glass flask whose volume is 1000.00 cm³ at 0.0°C is completely filled with mercury at this temper- ature. When the flask and the mercury are warmed to 55.0°C, 8.95 cm³ of mercury overflow. If the coefficient of volume expansion of mercury is 18.0 x 105 K-1, what is the coefficient of expansion of the glass?arrow_forward
- At a temperature of 22.90°C the hole in a steel plate has a diameter of 1.025 cm. If a steel rod with a diameter of 1.034 cm has to just slip through this hole, to what temperature should the plate be heated? The coefficient of area expansion of steel is 24 x 10-6/°c. °Carrow_forward3. A steel rod has a length of 3.000 cm at room temperature (20° C). A brass rod has a length of 2.997 cm at the same temperature. To what temperature must the rods be heated so that both of them will have the same length. (Coeffcients of linear expansion of steel and brass are, respectively, 11 x 10-6 K-¹ and 19 × 10-6 K-¹.)arrow_forward6. A Goodyear blimp typically contains 4900 m of helium (He) at an absolute pressure of 1.00 x 10* Pa. The temperature of the helium is 275 K. What is the mass (in kg) of the helium in the blimp?(The molar mass of Helium is 4.0026 g) kgarrow_forward
- 39. A tanker ship is filled with 2.25 x 105 m3 of gasoline at a refinery in southern Texas when the temperature is 17.2 °C. When the ship arrives in New York City, the temperature is 1.3 °C. If the coefficient of volumetric expansion for gasoline is 9.50 x 104/C°, how much has the volume of the gasoline decreased when it is unloaded in New York? A) 1.50 x 10-2 m3 B) 66.2 m3 C) 1290 m D) 3400 m3 E) 1.05 x 104 m3 40. Which one of the following statements best explains why gases are not commercially sold by volume? A) Gas volume is negligible. B) Gas volume is difficult to measure. C) Gas volume depends on the type of gas. D) Gases have comparatively low densities. E) Gas volume depends on temperature and pressure.arrow_forward2. A student measures the length of a brass rod at 20.0°C. The reading is 95.00 cm. What will be the length of the rod when the temperature is at: (a) - 15.0°C? (5 pts) (b) 55.0°C? (5 pts)arrow_forward7. A cube of ice is taken from the freezer at -14 °C and placed in a 130 g aluminum calorimeter filled with 350 g of water at a temperature of 26 °C. The final situation is observed to be all water at 14 °C. What was the mass of the ice cube? (Cwater-4186 J/kg. °C, Cal 900 J/kg.°C, Cice-2000 J/kg. °C, L=3.35 × 10 J/kg) grams #3 80 F3 E $ 4 000 F4 R DE F % 5 F5 T ● G ^ 6 MacBook Air B F6 Y H & 7 F7 U N * 0 8 J DII F8 M 9 K 2 F9 O < L F10 P J F11 + F12 8 100 ? 1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios